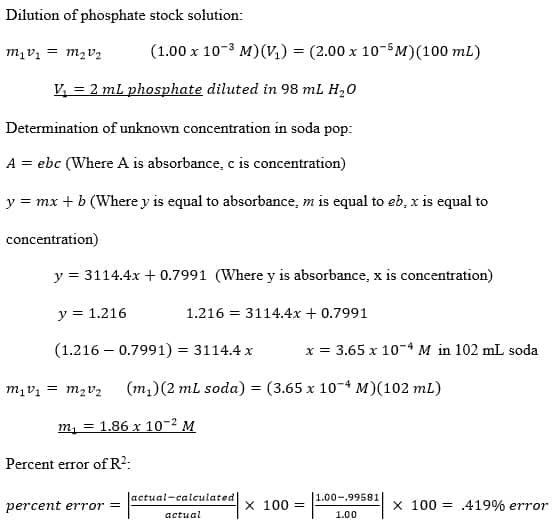
Dilution Vs Absorbance . according to beer's law, a = εlc, a substance's concentration and absorbance are directly proportional under ideal conditions: the overall goal of this lab was to make a calibration curve with a plot of absorbance vs. calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Describe how the concentration of a sample. Concentration and be able to determine the phosphate concentrations in samples of cola, surface water, and other aqueous solutions of if a solution is too concentrated (a>1) you simply need to dilute it until it is in the correct range and use the dilution factor to back calculate the. Determine order of reaction by. make a stock solution of the appropriate concentration. describe how the transmission and absorption of light relates to the appearance of solutions. Create a series of solutions of decreasing concentrations via serial dilutions.
from schoolworkhelper.net
Determine order of reaction by. Describe how the concentration of a sample. describe how the transmission and absorption of light relates to the appearance of solutions. the overall goal of this lab was to make a calibration curve with a plot of absorbance vs. calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Concentration and be able to determine the phosphate concentrations in samples of cola, surface water, and other aqueous solutions of according to beer's law, a = εlc, a substance's concentration and absorbance are directly proportional under ideal conditions: Create a series of solutions of decreasing concentrations via serial dilutions. make a stock solution of the appropriate concentration. if a solution is too concentrated (a>1) you simply need to dilute it until it is in the correct range and use the dilution factor to back calculate the.
Beer’s Law Lab Explained Absorbance vs. Concentration SchoolWorkHelper
Dilution Vs Absorbance Create a series of solutions of decreasing concentrations via serial dilutions. describe how the transmission and absorption of light relates to the appearance of solutions. calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Determine order of reaction by. Create a series of solutions of decreasing concentrations via serial dilutions. the overall goal of this lab was to make a calibration curve with a plot of absorbance vs. make a stock solution of the appropriate concentration. Describe how the concentration of a sample. if a solution is too concentrated (a>1) you simply need to dilute it until it is in the correct range and use the dilution factor to back calculate the. Concentration and be able to determine the phosphate concentrations in samples of cola, surface water, and other aqueous solutions of according to beer's law, a = εlc, a substance's concentration and absorbance are directly proportional under ideal conditions:
From www.researchgate.net
Absorbance vs time Dilution factor= 2, glucose concentration= 50 mg Dilution Vs Absorbance Determine order of reaction by. Describe how the concentration of a sample. make a stock solution of the appropriate concentration. according to beer's law, a = εlc, a substance's concentration and absorbance are directly proportional under ideal conditions: calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). describe how the transmission and. Dilution Vs Absorbance.
From chart-studio.plotly.com
Graph 1 umoles pNP vs Absorbance scatter chart made by Jkuhn plotly Dilution Vs Absorbance Concentration and be able to determine the phosphate concentrations in samples of cola, surface water, and other aqueous solutions of calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Describe how the concentration of a sample. Create a series of solutions of decreasing concentrations via serial dilutions. if a solution is too concentrated (a>1). Dilution Vs Absorbance.
From chart-studio.plotly.com
Absorbance vs Dilution factor scatter chart made by Bigkendog plotly Dilution Vs Absorbance Determine order of reaction by. the overall goal of this lab was to make a calibration curve with a plot of absorbance vs. Create a series of solutions of decreasing concentrations via serial dilutions. describe how the transmission and absorption of light relates to the appearance of solutions. if a solution is too concentrated (a>1) you simply. Dilution Vs Absorbance.
From exyzklqnz.blob.core.windows.net
Dilution Calculation Equations at Rebecca Mathis blog Dilution Vs Absorbance make a stock solution of the appropriate concentration. the overall goal of this lab was to make a calibration curve with a plot of absorbance vs. describe how the transmission and absorption of light relates to the appearance of solutions. Describe how the concentration of a sample. according to beer's law, a = εlc, a substance's. Dilution Vs Absorbance.
From exyxdeytx.blob.core.windows.net
Dilution Absorbance Calculation at Clyde Rodrigues blog Dilution Vs Absorbance the overall goal of this lab was to make a calibration curve with a plot of absorbance vs. Determine order of reaction by. describe how the transmission and absorption of light relates to the appearance of solutions. according to beer's law, a = εlc, a substance's concentration and absorbance are directly proportional under ideal conditions: calculate. Dilution Vs Absorbance.
From www.researchgate.net
Gradient of absorbance vs. concentration graph for gallic acid Dilution Vs Absorbance make a stock solution of the appropriate concentration. calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). if a solution is too concentrated (a>1) you simply need to dilute it until it is in the correct range and use the dilution factor to back calculate the. the overall goal of this lab. Dilution Vs Absorbance.
From www.vernier.com
Decoding Your Absorbance Readings Vernier Dilution Vs Absorbance Concentration and be able to determine the phosphate concentrations in samples of cola, surface water, and other aqueous solutions of Describe how the concentration of a sample. make a stock solution of the appropriate concentration. according to beer's law, a = εlc, a substance's concentration and absorbance are directly proportional under ideal conditions: Determine order of reaction by.. Dilution Vs Absorbance.
From www.researchgate.net
Concentration vs. absorbance curve generated from data collected using Dilution Vs Absorbance Create a series of solutions of decreasing concentrations via serial dilutions. make a stock solution of the appropriate concentration. calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). describe how the transmission and absorption of light relates to the appearance of solutions. if a solution is too concentrated (a>1) you simply need. Dilution Vs Absorbance.
From exyxdeytx.blob.core.windows.net
Dilution Absorbance Calculation at Clyde Rodrigues blog Dilution Vs Absorbance if a solution is too concentrated (a>1) you simply need to dilute it until it is in the correct range and use the dilution factor to back calculate the. describe how the transmission and absorption of light relates to the appearance of solutions. according to beer's law, a = εlc, a substance's concentration and absorbance are directly. Dilution Vs Absorbance.
From schoolworkhelper.net
Beer’s Law Lab Explained Absorbance vs. Concentration SchoolWorkHelper Dilution Vs Absorbance describe how the transmission and absorption of light relates to the appearance of solutions. Determine order of reaction by. according to beer's law, a = εlc, a substance's concentration and absorbance are directly proportional under ideal conditions: Create a series of solutions of decreasing concentrations via serial dilutions. Describe how the concentration of a sample. if a. Dilution Vs Absorbance.
From www.researchgate.net
Why do antigen concentrations measured in ELISA change depending on Dilution Vs Absorbance according to beer's law, a = εlc, a substance's concentration and absorbance are directly proportional under ideal conditions: Concentration and be able to determine the phosphate concentrations in samples of cola, surface water, and other aqueous solutions of the overall goal of this lab was to make a calibration curve with a plot of absorbance vs. Describe how. Dilution Vs Absorbance.
From moniqueaafiyah.blogspot.com
Share dilution calculator MoniqueAafiyah Dilution Vs Absorbance Determine order of reaction by. calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Create a series of solutions of decreasing concentrations via serial dilutions. the overall goal of this lab was to make a calibration curve with a plot of absorbance vs. if a solution is too concentrated (a>1) you simply need. Dilution Vs Absorbance.
From definitionhjo.blogspot.com
Definition Of Absorbance In Chemistry DEFINITION HJO Dilution Vs Absorbance the overall goal of this lab was to make a calibration curve with a plot of absorbance vs. Create a series of solutions of decreasing concentrations via serial dilutions. Determine order of reaction by. describe how the transmission and absorption of light relates to the appearance of solutions. Describe how the concentration of a sample. Concentration and be. Dilution Vs Absorbance.
From www.researchgate.net
The concentration vs. absorbance curves generated from known Dilution Vs Absorbance Determine order of reaction by. Concentration and be able to determine the phosphate concentrations in samples of cola, surface water, and other aqueous solutions of according to beer's law, a = εlc, a substance's concentration and absorbance are directly proportional under ideal conditions: the overall goal of this lab was to make a calibration curve with a plot. Dilution Vs Absorbance.
From rheolution.com
Understanding Absorbance at Specific Wavelengths Dilution Vs Absorbance Describe how the concentration of a sample. the overall goal of this lab was to make a calibration curve with a plot of absorbance vs. Concentration and be able to determine the phosphate concentrations in samples of cola, surface water, and other aqueous solutions of according to beer's law, a = εlc, a substance's concentration and absorbance are. Dilution Vs Absorbance.
From www.researchgate.net
Absorbance spectra of 150 dilutions of dye solution 1 after Dilution Vs Absorbance Determine order of reaction by. if a solution is too concentrated (a>1) you simply need to dilute it until it is in the correct range and use the dilution factor to back calculate the. the overall goal of this lab was to make a calibration curve with a plot of absorbance vs. Concentration and be able to determine. Dilution Vs Absorbance.
From www.researchgate.net
Standard calibration curve of Dglucose (absorbance measured at 489 nm Dilution Vs Absorbance the overall goal of this lab was to make a calibration curve with a plot of absorbance vs. if a solution is too concentrated (a>1) you simply need to dilute it until it is in the correct range and use the dilution factor to back calculate the. Create a series of solutions of decreasing concentrations via serial dilutions.. Dilution Vs Absorbance.
From www.openwetware.org
UserKlare Lazor/Notebook/Chem496001/2011/09/14 OpenWetWare Dilution Vs Absorbance make a stock solution of the appropriate concentration. Determine order of reaction by. describe how the transmission and absorption of light relates to the appearance of solutions. calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). if a solution is too concentrated (a>1) you simply need to dilute it until it is. Dilution Vs Absorbance.
From www.researchgate.net
Graph of Absorbance vs Concentration Download Scientific Diagram Dilution Vs Absorbance Determine order of reaction by. Create a series of solutions of decreasing concentrations via serial dilutions. Concentration and be able to determine the phosphate concentrations in samples of cola, surface water, and other aqueous solutions of calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). the overall goal of this lab was to make. Dilution Vs Absorbance.
From www.slideshare.net
Serial dilution Dilution Vs Absorbance calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). describe how the transmission and absorption of light relates to the appearance of solutions. Describe how the concentration of a sample. if a solution is too concentrated (a>1) you simply need to dilute it until it is in the correct range and use the. Dilution Vs Absorbance.
From www.vrogue.co
What Is The Difference Between Trademark Dilution Vs vrogue.co Dilution Vs Absorbance Describe how the concentration of a sample. if a solution is too concentrated (a>1) you simply need to dilute it until it is in the correct range and use the dilution factor to back calculate the. Determine order of reaction by. describe how the transmission and absorption of light relates to the appearance of solutions. Concentration and be. Dilution Vs Absorbance.
From rheolution.com
Understanding Absorbance at Specific Wavelengths Dilution Vs Absorbance Describe how the concentration of a sample. if a solution is too concentrated (a>1) you simply need to dilute it until it is in the correct range and use the dilution factor to back calculate the. according to beer's law, a = εlc, a substance's concentration and absorbance are directly proportional under ideal conditions: calculate the concentration. Dilution Vs Absorbance.
From exovmtirc.blob.core.windows.net
Dilution Vs. Absorption at Charlie Goings blog Dilution Vs Absorbance Determine order of reaction by. describe how the transmission and absorption of light relates to the appearance of solutions. Create a series of solutions of decreasing concentrations via serial dilutions. the overall goal of this lab was to make a calibration curve with a plot of absorbance vs. make a stock solution of the appropriate concentration. Describe. Dilution Vs Absorbance.
From exyxdeytx.blob.core.windows.net
Dilution Absorbance Calculation at Clyde Rodrigues blog Dilution Vs Absorbance Create a series of solutions of decreasing concentrations via serial dilutions. if a solution is too concentrated (a>1) you simply need to dilute it until it is in the correct range and use the dilution factor to back calculate the. according to beer's law, a = εlc, a substance's concentration and absorbance are directly proportional under ideal conditions:. Dilution Vs Absorbance.
From fyozjpalw.blob.core.windows.net
Number Of Cells In Dilution at Brad Bray blog Dilution Vs Absorbance calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Determine order of reaction by. make a stock solution of the appropriate concentration. if a solution is too concentrated (a>1) you simply need to dilute it until it is in the correct range and use the dilution factor to back calculate the. the. Dilution Vs Absorbance.
From giomjtpgc.blob.core.windows.net
Dilution Ratio Definition at Juan Hambright blog Dilution Vs Absorbance make a stock solution of the appropriate concentration. describe how the transmission and absorption of light relates to the appearance of solutions. Concentration and be able to determine the phosphate concentrations in samples of cola, surface water, and other aqueous solutions of according to beer's law, a = εlc, a substance's concentration and absorbance are directly proportional. Dilution Vs Absorbance.
From www.researchgate.net
Concentration vs. Absorbance for reactive yellow dye Download Dilution Vs Absorbance Concentration and be able to determine the phosphate concentrations in samples of cola, surface water, and other aqueous solutions of Determine order of reaction by. the overall goal of this lab was to make a calibration curve with a plot of absorbance vs. if a solution is too concentrated (a>1) you simply need to dilute it until it. Dilution Vs Absorbance.
From www.researchgate.net
The relationship between absorbance measured on a spectrophotometer Dilution Vs Absorbance the overall goal of this lab was to make a calibration curve with a plot of absorbance vs. according to beer's law, a = εlc, a substance's concentration and absorbance are directly proportional under ideal conditions: calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). Describe how the concentration of a sample. Concentration. Dilution Vs Absorbance.
From www.researchgate.net
Absorbance vs concentration graph at 462 nm, 0100 dilutions of Dilution Vs Absorbance calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). the overall goal of this lab was to make a calibration curve with a plot of absorbance vs. Describe how the concentration of a sample. if a solution is too concentrated (a>1) you simply need to dilute it until it is in the correct. Dilution Vs Absorbance.
From chart-studio.plotly.com
Dilution vs. Optical Density (686 nm) scatter chart made by Dilution Vs Absorbance describe how the transmission and absorption of light relates to the appearance of solutions. Determine order of reaction by. make a stock solution of the appropriate concentration. if a solution is too concentrated (a>1) you simply need to dilute it until it is in the correct range and use the dilution factor to back calculate the. Concentration. Dilution Vs Absorbance.
From exyzyeegq.blob.core.windows.net
Graph Of Absorbance Vs Time at Henry Johnson blog Dilution Vs Absorbance describe how the transmission and absorption of light relates to the appearance of solutions. the overall goal of this lab was to make a calibration curve with a plot of absorbance vs. calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). according to beer's law, a = εlc, a substance's concentration and. Dilution Vs Absorbance.
From www.researchgate.net
(A) DA concentrations versus the corresponding ELISA absorbance values Dilution Vs Absorbance if a solution is too concentrated (a>1) you simply need to dilute it until it is in the correct range and use the dilution factor to back calculate the. Concentration and be able to determine the phosphate concentrations in samples of cola, surface water, and other aqueous solutions of make a stock solution of the appropriate concentration. Create. Dilution Vs Absorbance.
From www.researchgate.net
NADH vs. NADPH calibration curve for enzyme assay interchangeable? Dilution Vs Absorbance Create a series of solutions of decreasing concentrations via serial dilutions. describe how the transmission and absorption of light relates to the appearance of solutions. Determine order of reaction by. according to beer's law, a = εlc, a substance's concentration and absorbance are directly proportional under ideal conditions: calculate the concentration of a dilute aqueous solute using. Dilution Vs Absorbance.
From abbymchem.blogspot.com
Abby McDonough AP Chem Blog Concentration, Molarity, Absorbance Dilution Vs Absorbance describe how the transmission and absorption of light relates to the appearance of solutions. if a solution is too concentrated (a>1) you simply need to dilute it until it is in the correct range and use the dilution factor to back calculate the. Create a series of solutions of decreasing concentrations via serial dilutions. make a stock. Dilution Vs Absorbance.
From www.numerade.com
SOLVED Experiment 1 Table 1 Absorbance vs wavelength of methylene Dilution Vs Absorbance calculate the concentration of a dilute aqueous solute using absorbance spectroscopy (beer's law). describe how the transmission and absorption of light relates to the appearance of solutions. Concentration and be able to determine the phosphate concentrations in samples of cola, surface water, and other aqueous solutions of Determine order of reaction by. according to beer's law, a. Dilution Vs Absorbance.