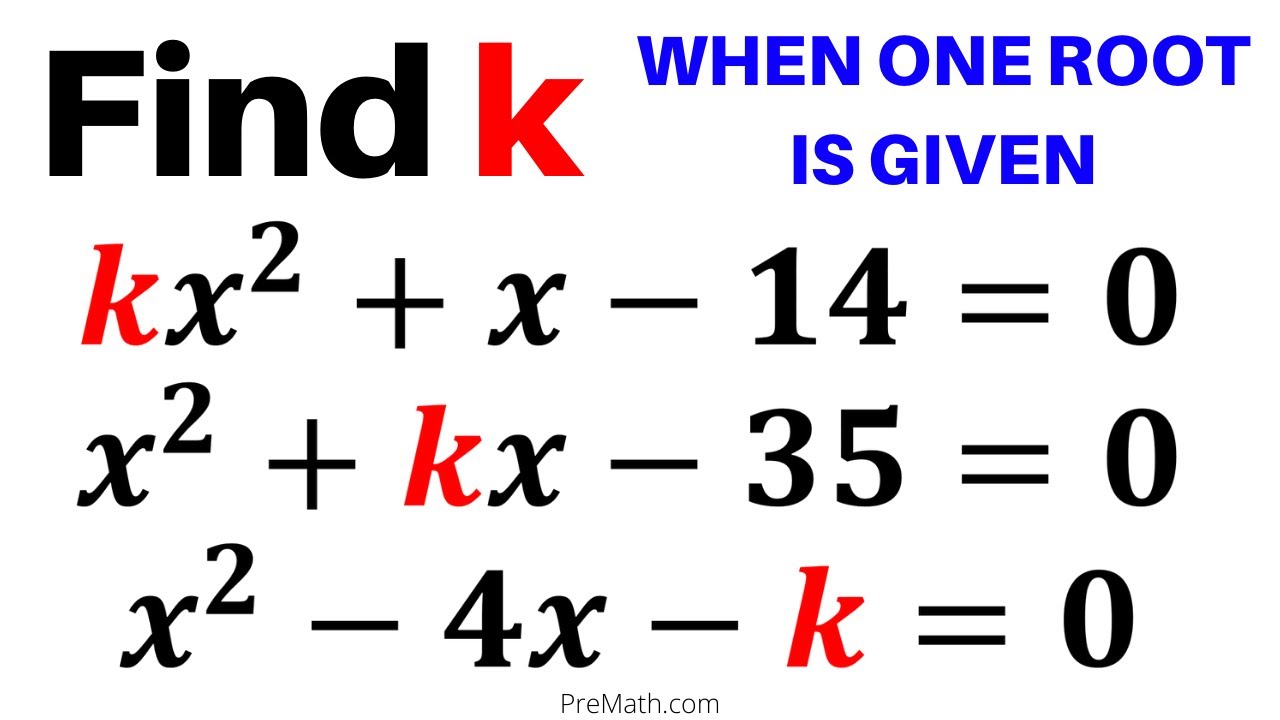How To Calculate The Value Of K . Arrange the equations so that their sum produces the overall equation. Write out the balanced chemical equation with the concentrations of beneath each substance using an initial, change and. Write the equilibrium constant expression for the reaction. Substitute the known k value and the final concentrations to solve for. Using the e x function on your calculator gives a value for k = 2.53 x 10 8. Pc × c p d. A small value means that. We need to know two things in order to calculate the numeric value of the equilibrium constant: The balanced equation for the reaction system,. If an equation had to be reversed, invert the value of \ (k\) for that equation. With this tool, you can calculate the value of an equilibrium constant for a reaction while learning how to calculate the equilibrium. That is a huge value for an equilibrium constant, and means that at. For gases, it is often more convenient to express the equilibrium constant in terms of partial pressures: A large value of the equilibrium constant \(k\) means that products predominate at equilibrium;
from www.youtube.com
For gases, it is often more convenient to express the equilibrium constant in terms of partial pressures: If an equation had to be reversed, invert the value of \ (k\) for that equation. We need to know two things in order to calculate the numeric value of the equilibrium constant: Write out the balanced chemical equation with the concentrations of beneath each substance using an initial, change and. The balanced equation for the reaction system,. Substitute the known k value and the final concentrations to solve for. That is a huge value for an equilibrium constant, and means that at. A small value means that. Using the e x function on your calculator gives a value for k = 2.53 x 10 8. Arrange the equations so that their sum produces the overall equation.
Find the Value of k in Quadratic Equations when One Root is Given
How To Calculate The Value Of K Arrange the equations so that their sum produces the overall equation. Write out the balanced chemical equation with the concentrations of beneath each substance using an initial, change and. Write the equilibrium constant expression for the reaction. Pc × c p d. We need to know two things in order to calculate the numeric value of the equilibrium constant: The balanced equation for the reaction system,. With this tool, you can calculate the value of an equilibrium constant for a reaction while learning how to calculate the equilibrium. Arrange the equations so that their sum produces the overall equation. If an equation had to be reversed, invert the value of \ (k\) for that equation. A small value means that. A large value of the equilibrium constant \(k\) means that products predominate at equilibrium; Using the e x function on your calculator gives a value for k = 2.53 x 10 8. Substitute the known k value and the final concentrations to solve for. That is a huge value for an equilibrium constant, and means that at. For gases, it is often more convenient to express the equilibrium constant in terms of partial pressures:
From games.udlvirtual.edu.pe
K Value Statistics BEST GAMES WALKTHROUGH How To Calculate The Value Of K With this tool, you can calculate the value of an equilibrium constant for a reaction while learning how to calculate the equilibrium. That is a huge value for an equilibrium constant, and means that at. Pc × c p d. A large value of the equilibrium constant \(k\) means that products predominate at equilibrium; Write the equilibrium constant expression for. How To Calculate The Value Of K.
From www.youtube.com
How to find value of k in linear equation? YouTube How To Calculate The Value Of K Substitute the known k value and the final concentrations to solve for. That is a huge value for an equilibrium constant, and means that at. Write out the balanced chemical equation with the concentrations of beneath each substance using an initial, change and. Pc × c p d. Using the e x function on your calculator gives a value for. How To Calculate The Value Of K.
From www.youtube.com
How to Find Value of K in Math Class 10th Chapter 3 Pair of How To Calculate The Value Of K Substitute the known k value and the final concentrations to solve for. Write the equilibrium constant expression for the reaction. Pc × c p d. That is a huge value for an equilibrium constant, and means that at. Write out the balanced chemical equation with the concentrations of beneath each substance using an initial, change and. Arrange the equations so. How To Calculate The Value Of K.
From www.youtube.com
Determine the rate constant (k) for a reaction YouTube How To Calculate The Value Of K For gases, it is often more convenient to express the equilibrium constant in terms of partial pressures: That is a huge value for an equilibrium constant, and means that at. Write out the balanced chemical equation with the concentrations of beneath each substance using an initial, change and. Arrange the equations so that their sum produces the overall equation. Pc. How To Calculate The Value Of K.
From www.youtube.com
How To Determine The Units Of The Rate Constant K Chemical How To Calculate The Value Of K Write out the balanced chemical equation with the concentrations of beneath each substance using an initial, change and. If an equation had to be reversed, invert the value of \ (k\) for that equation. A small value means that. We need to know two things in order to calculate the numeric value of the equilibrium constant: Pc × c p. How To Calculate The Value Of K.
From www.teachoo.com
Find value of k, for which one root quadratic equation kx2 14x + 8=0 How To Calculate The Value Of K Write out the balanced chemical equation with the concentrations of beneath each substance using an initial, change and. A large value of the equilibrium constant \(k\) means that products predominate at equilibrium; Write the equilibrium constant expression for the reaction. If an equation had to be reversed, invert the value of \ (k\) for that equation. Arrange the equations so. How To Calculate The Value Of K.
From www.teachoo.com
Ex 4.4, 2 Find values of k if equation has two equal roots How To Calculate The Value Of K Pc × c p d. Using the e x function on your calculator gives a value for k = 2.53 x 10 8. A large value of the equilibrium constant \(k\) means that products predominate at equilibrium; With this tool, you can calculate the value of an equilibrium constant for a reaction while learning how to calculate the equilibrium. That. How To Calculate The Value Of K.
From www.tessshebaylo.com
How To Find Value Of K In Quadratic Equation Tessshebaylo How To Calculate The Value Of K The balanced equation for the reaction system,. That is a huge value for an equilibrium constant, and means that at. A large value of the equilibrium constant \(k\) means that products predominate at equilibrium; With this tool, you can calculate the value of an equilibrium constant for a reaction while learning how to calculate the equilibrium. Using the e x. How To Calculate The Value Of K.
From www.youtube.com
Chemical Equilibrium Constant K Ice Tables Kp and Kc Membership How To Calculate The Value Of K A large value of the equilibrium constant \(k\) means that products predominate at equilibrium; Arrange the equations so that their sum produces the overall equation. A small value means that. With this tool, you can calculate the value of an equilibrium constant for a reaction while learning how to calculate the equilibrium. Write the equilibrium constant expression for the reaction.. How To Calculate The Value Of K.
From www.youtube.com
Find value of k to make polynomials perfect square trinomial. 4x^2 kx How To Calculate The Value Of K With this tool, you can calculate the value of an equilibrium constant for a reaction while learning how to calculate the equilibrium. Write the equilibrium constant expression for the reaction. We need to know two things in order to calculate the numeric value of the equilibrium constant: A small value means that. Pc × c p d. Arrange the equations. How To Calculate The Value Of K.
From www.youtube.com
How to find the value of K? YouTube How To Calculate The Value Of K If an equation had to be reversed, invert the value of \ (k\) for that equation. Arrange the equations so that their sum produces the overall equation. We need to know two things in order to calculate the numeric value of the equilibrium constant: Using the e x function on your calculator gives a value for k = 2.53 x. How To Calculate The Value Of K.
From www.chegg.com
Solved Find the value of k for which the matrix A = [8 8 16 How To Calculate The Value Of K If an equation had to be reversed, invert the value of \ (k\) for that equation. That is a huge value for an equilibrium constant, and means that at. Arrange the equations so that their sum produces the overall equation. We need to know two things in order to calculate the numeric value of the equilibrium constant: Using the e. How To Calculate The Value Of K.
From www.youtube.com
Find the value of k that will make relation a function. {(3,2), (4,9 How To Calculate The Value Of K That is a huge value for an equilibrium constant, and means that at. Arrange the equations so that their sum produces the overall equation. With this tool, you can calculate the value of an equilibrium constant for a reaction while learning how to calculate the equilibrium. A small value means that. Substitute the known k value and the final concentrations. How To Calculate The Value Of K.
From www.tessshebaylo.com
How To Find The Value Of K In A Quadratic Equation With Equal Roots How To Calculate The Value Of K We need to know two things in order to calculate the numeric value of the equilibrium constant: For gases, it is often more convenient to express the equilibrium constant in terms of partial pressures: If an equation had to be reversed, invert the value of \ (k\) for that equation. Write out the balanced chemical equation with the concentrations of. How To Calculate The Value Of K.
From www.tessshebaylo.com
How To Find The Value Of K In A Quadratic Equation With Equal Roots How To Calculate The Value Of K Arrange the equations so that their sum produces the overall equation. We need to know two things in order to calculate the numeric value of the equilibrium constant: A large value of the equilibrium constant \(k\) means that products predominate at equilibrium; Substitute the known k value and the final concentrations to solve for. If an equation had to be. How To Calculate The Value Of K.
From www.tessshebaylo.com
How To Find The Value Of K In A Quadratic Equation With Equal Roots How To Calculate The Value Of K If an equation had to be reversed, invert the value of \ (k\) for that equation. Arrange the equations so that their sum produces the overall equation. For gases, it is often more convenient to express the equilibrium constant in terms of partial pressures: With this tool, you can calculate the value of an equilibrium constant for a reaction while. How To Calculate The Value Of K.
From www.tessshebaylo.com
How To Find Value Of K In Quadratic Equation Tessshebaylo How To Calculate The Value Of K Substitute the known k value and the final concentrations to solve for. The balanced equation for the reaction system,. Write the equilibrium constant expression for the reaction. Arrange the equations so that their sum produces the overall equation. With this tool, you can calculate the value of an equilibrium constant for a reaction while learning how to calculate the equilibrium.. How To Calculate The Value Of K.
From www.youtube.com
Calculating K from Concentration YouTube How To Calculate The Value Of K If an equation had to be reversed, invert the value of \ (k\) for that equation. Using the e x function on your calculator gives a value for k = 2.53 x 10 8. With this tool, you can calculate the value of an equilibrium constant for a reaction while learning how to calculate the equilibrium. A small value means. How To Calculate The Value Of K.
From www.youtube.com
How to Calculate Equilibrium Constant K Value Practice Problems How To Calculate The Value Of K That is a huge value for an equilibrium constant, and means that at. Write the equilibrium constant expression for the reaction. We need to know two things in order to calculate the numeric value of the equilibrium constant: Pc × c p d. If an equation had to be reversed, invert the value of \ (k\) for that equation. For. How To Calculate The Value Of K.
From www.teachoo.com
What is Minimum and Maximum Value of Investment Multipler k? How To Calculate The Value Of K Pc × c p d. Using the e x function on your calculator gives a value for k = 2.53 x 10 8. For gases, it is often more convenient to express the equilibrium constant in terms of partial pressures: With this tool, you can calculate the value of an equilibrium constant for a reaction while learning how to calculate. How To Calculate The Value Of K.
From lessonbergininviting.z21.web.core.windows.net
How To Find K In Algebra How To Calculate The Value Of K With this tool, you can calculate the value of an equilibrium constant for a reaction while learning how to calculate the equilibrium. If an equation had to be reversed, invert the value of \ (k\) for that equation. The balanced equation for the reaction system,. Substitute the known k value and the final concentrations to solve for. A large value. How To Calculate The Value Of K.
From feevalue.com
find the value of k so that the quadratic equation If the equation (k+1 How To Calculate The Value Of K Pc × c p d. With this tool, you can calculate the value of an equilibrium constant for a reaction while learning how to calculate the equilibrium. A large value of the equilibrium constant \(k\) means that products predominate at equilibrium; Write the equilibrium constant expression for the reaction. That is a huge value for an equilibrium constant, and means. How To Calculate The Value Of K.
From www.youtube.com
FIND THE VALUE OF K THAT MAKES THE FUNCTION CONTINUOUS YouTube How To Calculate The Value Of K We need to know two things in order to calculate the numeric value of the equilibrium constant: If an equation had to be reversed, invert the value of \ (k\) for that equation. With this tool, you can calculate the value of an equilibrium constant for a reaction while learning how to calculate the equilibrium. A large value of the. How To Calculate The Value Of K.
From www.toppr.com
For what value of k, the matrix 2 k 3 5 1 is not invertible? How To Calculate The Value Of K We need to know two things in order to calculate the numeric value of the equilibrium constant: Pc × c p d. A large value of the equilibrium constant \(k\) means that products predominate at equilibrium; Using the e x function on your calculator gives a value for k = 2.53 x 10 8. For gases, it is often more. How To Calculate The Value Of K.
From www.youtube.com
Find the Value of K to make a Perfect Square Trinomial StepbyStep How To Calculate The Value Of K A large value of the equilibrium constant \(k\) means that products predominate at equilibrium; The balanced equation for the reaction system,. Arrange the equations so that their sum produces the overall equation. If an equation had to be reversed, invert the value of \ (k\) for that equation. Using the e x function on your calculator gives a value for. How To Calculate The Value Of K.
From haipernews.com
How To Calculate Equilibrium Constant K Haiper How To Calculate The Value Of K Arrange the equations so that their sum produces the overall equation. For gases, it is often more convenient to express the equilibrium constant in terms of partial pressures: Write out the balanced chemical equation with the concentrations of beneath each substance using an initial, change and. A small value means that. A large value of the equilibrium constant \(k\) means. How To Calculate The Value Of K.
From www.tessshebaylo.com
How To Find The Value Of K In A Quadratic Equation With Equal Roots How To Calculate The Value Of K Using the e x function on your calculator gives a value for k = 2.53 x 10 8. Write out the balanced chemical equation with the concentrations of beneath each substance using an initial, change and. Substitute the known k value and the final concentrations to solve for. If an equation had to be reversed, invert the value of \. How To Calculate The Value Of K.
From mungfali.com
Find Values Of K For Each Of The Following Quadratic Equations So That 652 How To Calculate The Value Of K With this tool, you can calculate the value of an equilibrium constant for a reaction while learning how to calculate the equilibrium. Arrange the equations so that their sum produces the overall equation. For gases, it is often more convenient to express the equilibrium constant in terms of partial pressures: Substitute the known k value and the final concentrations to. How To Calculate The Value Of K.
From www.tessshebaylo.com
How To Find Value Of K In Quadratic Equation Tessshebaylo How To Calculate The Value Of K For gases, it is often more convenient to express the equilibrium constant in terms of partial pressures: Pc × c p d. A large value of the equilibrium constant \(k\) means that products predominate at equilibrium; A small value means that. Write out the balanced chemical equation with the concentrations of beneath each substance using an initial, change and. That. How To Calculate The Value Of K.
From www.youtube.com
Find the Value of k in Quadratic Equations when One Root is Given How To Calculate The Value Of K Substitute the known k value and the final concentrations to solve for. Pc × c p d. We need to know two things in order to calculate the numeric value of the equilibrium constant: Using the e x function on your calculator gives a value for k = 2.53 x 10 8. Write the equilibrium constant expression for the reaction.. How To Calculate The Value Of K.
From www.youtube.com
Find k for Probability Distribution. Calculate the mean for this How To Calculate The Value Of K With this tool, you can calculate the value of an equilibrium constant for a reaction while learning how to calculate the equilibrium. The balanced equation for the reaction system,. A small value means that. If an equation had to be reversed, invert the value of \ (k\) for that equation. Arrange the equations so that their sum produces the overall. How To Calculate The Value Of K.
From www.youtube.com
Determine the Value of K for a Random Variable Distribution IB SL Test How To Calculate The Value Of K With this tool, you can calculate the value of an equilibrium constant for a reaction while learning how to calculate the equilibrium. Pc × c p d. For gases, it is often more convenient to express the equilibrium constant in terms of partial pressures: A large value of the equilibrium constant \(k\) means that products predominate at equilibrium; A small. How To Calculate The Value Of K.
From www.tessshebaylo.com
How To Find The Value Of K In A Quadratic Equation With Equal Roots How To Calculate The Value Of K Pc × c p d. With this tool, you can calculate the value of an equilibrium constant for a reaction while learning how to calculate the equilibrium. The balanced equation for the reaction system,. For gases, it is often more convenient to express the equilibrium constant in terms of partial pressures: Substitute the known k value and the final concentrations. How To Calculate The Value Of K.
From www.tessshebaylo.com
How To Find The Value Of K In A Quadratic Equation With Equal Roots How To Calculate The Value Of K The balanced equation for the reaction system,. Write the equilibrium constant expression for the reaction. That is a huge value for an equilibrium constant, and means that at. Substitute the known k value and the final concentrations to solve for. We need to know two things in order to calculate the numeric value of the equilibrium constant: Using the e. How To Calculate The Value Of K.
From www.youtube.com
Find the Value of k in Quadratics for Different Scenarios Involving How To Calculate The Value Of K A large value of the equilibrium constant \(k\) means that products predominate at equilibrium; Substitute the known k value and the final concentrations to solve for. Pc × c p d. For gases, it is often more convenient to express the equilibrium constant in terms of partial pressures: That is a huge value for an equilibrium constant, and means that. How To Calculate The Value Of K.