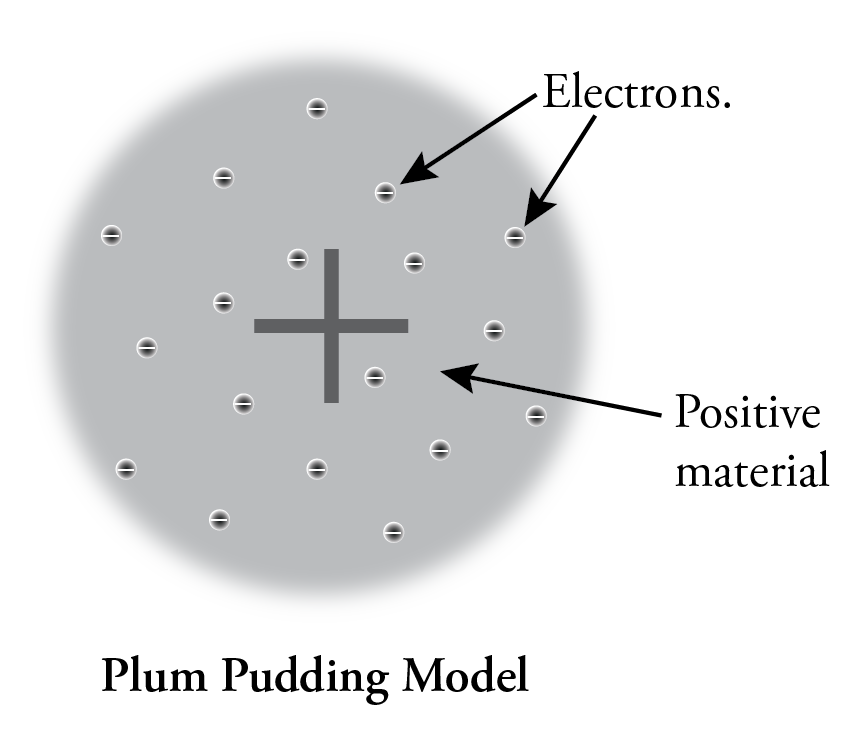
What Is The Plum Pudding Experiment . His two students, hans geiger and ernest marsden, directed a beam of. Plum pudding is an english dessert similar to a blueberry muffin. In thomson's plum pudding model of the atom, the electrons were embedded in a uniform sphere of positive. In 1905, ernest rutherford did an experiment to test the plum pudding model. The electrons were the negative plums embedded in a positive. After discovering the electron in 1897, j j thomson proposed that the atom looked like a. The model, british physicists recognized, was reminiscent of a plum pudding, a dessert adored by the british. Popularly known as the plum pudding model, it had to be abandoned (1911) on both theoretical and experimental grounds in favour of the rutherford atomic model, in. At a very thin gold.
from www.preparatorychemistry.com
In 1905, ernest rutherford did an experiment to test the plum pudding model. In thomson's plum pudding model of the atom, the electrons were embedded in a uniform sphere of positive. At a very thin gold. After discovering the electron in 1897, j j thomson proposed that the atom looked like a. The model, british physicists recognized, was reminiscent of a plum pudding, a dessert adored by the british. Popularly known as the plum pudding model, it had to be abandoned (1911) on both theoretical and experimental grounds in favour of the rutherford atomic model, in. Plum pudding is an english dessert similar to a blueberry muffin. His two students, hans geiger and ernest marsden, directed a beam of. The electrons were the negative plums embedded in a positive.
History of Atomic Theory
What Is The Plum Pudding Experiment The model, british physicists recognized, was reminiscent of a plum pudding, a dessert adored by the british. His two students, hans geiger and ernest marsden, directed a beam of. The electrons were the negative plums embedded in a positive. In thomson's plum pudding model of the atom, the electrons were embedded in a uniform sphere of positive. Plum pudding is an english dessert similar to a blueberry muffin. At a very thin gold. In 1905, ernest rutherford did an experiment to test the plum pudding model. After discovering the electron in 1897, j j thomson proposed that the atom looked like a. The model, british physicists recognized, was reminiscent of a plum pudding, a dessert adored by the british. Popularly known as the plum pudding model, it had to be abandoned (1911) on both theoretical and experimental grounds in favour of the rutherford atomic model, in.
From www.pinterest.com
The Atom Chemistry Is My Jam! Plum pudding model, Plum pudding, Atom What Is The Plum Pudding Experiment His two students, hans geiger and ernest marsden, directed a beam of. In thomson's plum pudding model of the atom, the electrons were embedded in a uniform sphere of positive. At a very thin gold. The model, british physicists recognized, was reminiscent of a plum pudding, a dessert adored by the british. Plum pudding is an english dessert similar to. What Is The Plum Pudding Experiment.
From studylib.net
Thomson, Plum Pudding Model, & Rutherford notes What Is The Plum Pudding Experiment After discovering the electron in 1897, j j thomson proposed that the atom looked like a. The model, british physicists recognized, was reminiscent of a plum pudding, a dessert adored by the british. His two students, hans geiger and ernest marsden, directed a beam of. Popularly known as the plum pudding model, it had to be abandoned (1911) on both. What Is The Plum Pudding Experiment.
From www.expii.com
Plum Pudding Model — Overview & Importance Expii What Is The Plum Pudding Experiment His two students, hans geiger and ernest marsden, directed a beam of. In 1905, ernest rutherford did an experiment to test the plum pudding model. The model, british physicists recognized, was reminiscent of a plum pudding, a dessert adored by the british. In thomson's plum pudding model of the atom, the electrons were embedded in a uniform sphere of positive.. What Is The Plum Pudding Experiment.
From www.zmescience.com
The Plum Pudding Model how a flawed idea was instrumental in our What Is The Plum Pudding Experiment Popularly known as the plum pudding model, it had to be abandoned (1911) on both theoretical and experimental grounds in favour of the rutherford atomic model, in. After discovering the electron in 1897, j j thomson proposed that the atom looked like a. In 1905, ernest rutherford did an experiment to test the plum pudding model. At a very thin. What Is The Plum Pudding Experiment.
From animalia-life.club
Thomsons Plum Pudding Atomic Model What Is The Plum Pudding Experiment In 1905, ernest rutherford did an experiment to test the plum pudding model. The electrons were the negative plums embedded in a positive. Popularly known as the plum pudding model, it had to be abandoned (1911) on both theoretical and experimental grounds in favour of the rutherford atomic model, in. The model, british physicists recognized, was reminiscent of a plum. What Is The Plum Pudding Experiment.
From www.slideserve.com
PPT History of Atomic Theory PowerPoint Presentation, free download What Is The Plum Pudding Experiment The electrons were the negative plums embedded in a positive. His two students, hans geiger and ernest marsden, directed a beam of. In 1905, ernest rutherford did an experiment to test the plum pudding model. The model, british physicists recognized, was reminiscent of a plum pudding, a dessert adored by the british. In thomson's plum pudding model of the atom,. What Is The Plum Pudding Experiment.
From getrecipecart.com
Traditional plum pudding Recipe Cart What Is The Plum Pudding Experiment Popularly known as the plum pudding model, it had to be abandoned (1911) on both theoretical and experimental grounds in favour of the rutherford atomic model, in. After discovering the electron in 1897, j j thomson proposed that the atom looked like a. At a very thin gold. In 1905, ernest rutherford did an experiment to test the plum pudding. What Is The Plum Pudding Experiment.
From www.youtube.com
Plum Pudding Model of Atom by JJ Thomson I Atomic Model YouTube What Is The Plum Pudding Experiment In thomson's plum pudding model of the atom, the electrons were embedded in a uniform sphere of positive. The model, british physicists recognized, was reminiscent of a plum pudding, a dessert adored by the british. His two students, hans geiger and ernest marsden, directed a beam of. After discovering the electron in 1897, j j thomson proposed that the atom. What Is The Plum Pudding Experiment.
From www.preparatorychemistry.com
History of Atomic Theory What Is The Plum Pudding Experiment His two students, hans geiger and ernest marsden, directed a beam of. The electrons were the negative plums embedded in a positive. In thomson's plum pudding model of the atom, the electrons were embedded in a uniform sphere of positive. In 1905, ernest rutherford did an experiment to test the plum pudding model. At a very thin gold. Popularly known. What Is The Plum Pudding Experiment.
From www.scienceabc.com
What Is JJ Thomson's Plum Pudding Model? What Is The Plum Pudding Experiment Popularly known as the plum pudding model, it had to be abandoned (1911) on both theoretical and experimental grounds in favour of the rutherford atomic model, in. After discovering the electron in 1897, j j thomson proposed that the atom looked like a. Plum pudding is an english dessert similar to a blueberry muffin. The model, british physicists recognized, was. What Is The Plum Pudding Experiment.
From www.slideserve.com
PPT The Development of Atomic Models PowerPoint Presentation, free What Is The Plum Pudding Experiment After discovering the electron in 1897, j j thomson proposed that the atom looked like a. His two students, hans geiger and ernest marsden, directed a beam of. The model, british physicists recognized, was reminiscent of a plum pudding, a dessert adored by the british. At a very thin gold. In 1905, ernest rutherford did an experiment to test the. What Is The Plum Pudding Experiment.
From www.visionlearning.com
Atomic Theory I Chemistry Visionlearning What Is The Plum Pudding Experiment His two students, hans geiger and ernest marsden, directed a beam of. At a very thin gold. In thomson's plum pudding model of the atom, the electrons were embedded in a uniform sphere of positive. The model, british physicists recognized, was reminiscent of a plum pudding, a dessert adored by the british. After discovering the electron in 1897, j j. What Is The Plum Pudding Experiment.
From slideplayer.com
Plum Pudding Model. ppt download What Is The Plum Pudding Experiment In 1905, ernest rutherford did an experiment to test the plum pudding model. Popularly known as the plum pudding model, it had to be abandoned (1911) on both theoretical and experimental grounds in favour of the rutherford atomic model, in. The model, british physicists recognized, was reminiscent of a plum pudding, a dessert adored by the british. Plum pudding is. What Is The Plum Pudding Experiment.
From www.youtube.com
Plum pudding model YouTube What Is The Plum Pudding Experiment The model, british physicists recognized, was reminiscent of a plum pudding, a dessert adored by the british. After discovering the electron in 1897, j j thomson proposed that the atom looked like a. His two students, hans geiger and ernest marsden, directed a beam of. At a very thin gold. Popularly known as the plum pudding model, it had to. What Is The Plum Pudding Experiment.
From animalia-life.club
Thomsons Plum Pudding Atomic Model What Is The Plum Pudding Experiment In thomson's plum pudding model of the atom, the electrons were embedded in a uniform sphere of positive. Plum pudding is an english dessert similar to a blueberry muffin. At a very thin gold. His two students, hans geiger and ernest marsden, directed a beam of. The electrons were the negative plums embedded in a positive. In 1905, ernest rutherford. What Is The Plum Pudding Experiment.
From www.youtube.com
JJ Thomson's Plum Pudding Model YouTube What Is The Plum Pudding Experiment At a very thin gold. Popularly known as the plum pudding model, it had to be abandoned (1911) on both theoretical and experimental grounds in favour of the rutherford atomic model, in. His two students, hans geiger and ernest marsden, directed a beam of. The electrons were the negative plums embedded in a positive. The model, british physicists recognized, was. What Is The Plum Pudding Experiment.
From www.slideserve.com
PPT RUTHERFORD’S EXPERIMENT PowerPoint Presentation, free download What Is The Plum Pudding Experiment Popularly known as the plum pudding model, it had to be abandoned (1911) on both theoretical and experimental grounds in favour of the rutherford atomic model, in. His two students, hans geiger and ernest marsden, directed a beam of. In thomson's plum pudding model of the atom, the electrons were embedded in a uniform sphere of positive. Plum pudding is. What Is The Plum Pudding Experiment.
From animalia-life.club
Thomsons Plum Pudding Atomic Model What Is The Plum Pudding Experiment In 1905, ernest rutherford did an experiment to test the plum pudding model. At a very thin gold. Plum pudding is an english dessert similar to a blueberry muffin. The electrons were the negative plums embedded in a positive. The model, british physicists recognized, was reminiscent of a plum pudding, a dessert adored by the british. After discovering the electron. What Is The Plum Pudding Experiment.
From www.youtube.com
Thomson’s Plum Pudding Model of The Atom GCSE Chemistry (91 What Is The Plum Pudding Experiment The model, british physicists recognized, was reminiscent of a plum pudding, a dessert adored by the british. The electrons were the negative plums embedded in a positive. His two students, hans geiger and ernest marsden, directed a beam of. Popularly known as the plum pudding model, it had to be abandoned (1911) on both theoretical and experimental grounds in favour. What Is The Plum Pudding Experiment.
From www.science-revision.co.uk
Atomic structure recap from gcse What Is The Plum Pudding Experiment At a very thin gold. Plum pudding is an english dessert similar to a blueberry muffin. His two students, hans geiger and ernest marsden, directed a beam of. Popularly known as the plum pudding model, it had to be abandoned (1911) on both theoretical and experimental grounds in favour of the rutherford atomic model, in. After discovering the electron in. What Is The Plum Pudding Experiment.
From www.expii.com
Plum Pudding Model — Overview & Importance Expii What Is The Plum Pudding Experiment The model, british physicists recognized, was reminiscent of a plum pudding, a dessert adored by the british. In thomson's plum pudding model of the atom, the electrons were embedded in a uniform sphere of positive. His two students, hans geiger and ernest marsden, directed a beam of. The electrons were the negative plums embedded in a positive. At a very. What Is The Plum Pudding Experiment.
From www.tes.com
Plum Pudding Model Teaching Resources What Is The Plum Pudding Experiment In 1905, ernest rutherford did an experiment to test the plum pudding model. Plum pudding is an english dessert similar to a blueberry muffin. Popularly known as the plum pudding model, it had to be abandoned (1911) on both theoretical and experimental grounds in favour of the rutherford atomic model, in. His two students, hans geiger and ernest marsden, directed. What Is The Plum Pudding Experiment.
From www.youtube.com
Plum pudding model meaning what is plum pudding model what does What Is The Plum Pudding Experiment His two students, hans geiger and ernest marsden, directed a beam of. The model, british physicists recognized, was reminiscent of a plum pudding, a dessert adored by the british. Popularly known as the plum pudding model, it had to be abandoned (1911) on both theoretical and experimental grounds in favour of the rutherford atomic model, in. At a very thin. What Is The Plum Pudding Experiment.
From www.teachoo.com
Plum Pudding Model of Atom (JJ Thomson's Model) Postulates, Limitati What Is The Plum Pudding Experiment After discovering the electron in 1897, j j thomson proposed that the atom looked like a. The model, british physicists recognized, was reminiscent of a plum pudding, a dessert adored by the british. In thomson's plum pudding model of the atom, the electrons were embedded in a uniform sphere of positive. At a very thin gold. The electrons were the. What Is The Plum Pudding Experiment.
From www.anyrgb.com
Rutherford scattering, GeigerMarsden experiment, hans Geiger, plum What Is The Plum Pudding Experiment The model, british physicists recognized, was reminiscent of a plum pudding, a dessert adored by the british. In 1905, ernest rutherford did an experiment to test the plum pudding model. In thomson's plum pudding model of the atom, the electrons were embedded in a uniform sphere of positive. Plum pudding is an english dessert similar to a blueberry muffin. Popularly. What Is The Plum Pudding Experiment.
From www.zmescience.com
The Plum Pudding Model how a flawed idea was instrumental in our What Is The Plum Pudding Experiment In thomson's plum pudding model of the atom, the electrons were embedded in a uniform sphere of positive. In 1905, ernest rutherford did an experiment to test the plum pudding model. The model, british physicists recognized, was reminiscent of a plum pudding, a dessert adored by the british. Plum pudding is an english dessert similar to a blueberry muffin. His. What Is The Plum Pudding Experiment.
From mungfali.com
Plum Pudding Model And Nuclear Model What Is The Plum Pudding Experiment His two students, hans geiger and ernest marsden, directed a beam of. At a very thin gold. In thomson's plum pudding model of the atom, the electrons were embedded in a uniform sphere of positive. Plum pudding is an english dessert similar to a blueberry muffin. The electrons were the negative plums embedded in a positive. Popularly known as the. What Is The Plum Pudding Experiment.
From www.nuclear-power.com
Thomson Model of the Atom Plum Pudding Model What Is The Plum Pudding Experiment In 1905, ernest rutherford did an experiment to test the plum pudding model. The electrons were the negative plums embedded in a positive. At a very thin gold. After discovering the electron in 1897, j j thomson proposed that the atom looked like a. Popularly known as the plum pudding model, it had to be abandoned (1911) on both theoretical. What Is The Plum Pudding Experiment.
From www.tasteofhome.com
Plum Pudding Recipe How to Make It What Is The Plum Pudding Experiment After discovering the electron in 1897, j j thomson proposed that the atom looked like a. At a very thin gold. His two students, hans geiger and ernest marsden, directed a beam of. Popularly known as the plum pudding model, it had to be abandoned (1911) on both theoretical and experimental grounds in favour of the rutherford atomic model, in.. What Is The Plum Pudding Experiment.
From www.slideshare.net
JJ Thomson The Plum Pudding Model What Is The Plum Pudding Experiment The electrons were the negative plums embedded in a positive. In 1905, ernest rutherford did an experiment to test the plum pudding model. His two students, hans geiger and ernest marsden, directed a beam of. At a very thin gold. In thomson's plum pudding model of the atom, the electrons were embedded in a uniform sphere of positive. Plum pudding. What Is The Plum Pudding Experiment.
From variosmodelo.blogspot.com
Describe Thomsons Plum Pudding Model Of The Atom Vários Modelos What Is The Plum Pudding Experiment His two students, hans geiger and ernest marsden, directed a beam of. Plum pudding is an english dessert similar to a blueberry muffin. In thomson's plum pudding model of the atom, the electrons were embedded in a uniform sphere of positive. The electrons were the negative plums embedded in a positive. Popularly known as the plum pudding model, it had. What Is The Plum Pudding Experiment.
From www.youtube.com
The Plum Pudding Atomic Model, J.J. Thomson and the Electron YouTube What Is The Plum Pudding Experiment His two students, hans geiger and ernest marsden, directed a beam of. In 1905, ernest rutherford did an experiment to test the plum pudding model. Popularly known as the plum pudding model, it had to be abandoned (1911) on both theoretical and experimental grounds in favour of the rutherford atomic model, in. In thomson's plum pudding model of the atom,. What Is The Plum Pudding Experiment.
From www.youtube.com
Rutherford Scattering and the Plum Pudding ModelPhET Simulation What Is The Plum Pudding Experiment In 1905, ernest rutherford did an experiment to test the plum pudding model. In thomson's plum pudding model of the atom, the electrons were embedded in a uniform sphere of positive. Plum pudding is an english dessert similar to a blueberry muffin. Popularly known as the plum pudding model, it had to be abandoned (1911) on both theoretical and experimental. What Is The Plum Pudding Experiment.
From www.breakingatom.com
Plum Pudding Model Definition What Is The Plum Pudding Experiment The model, british physicists recognized, was reminiscent of a plum pudding, a dessert adored by the british. Popularly known as the plum pudding model, it had to be abandoned (1911) on both theoretical and experimental grounds in favour of the rutherford atomic model, in. In 1905, ernest rutherford did an experiment to test the plum pudding model. Plum pudding is. What Is The Plum Pudding Experiment.
From mungfali.com
Thomson Plum Pudding Model What Is The Plum Pudding Experiment His two students, hans geiger and ernest marsden, directed a beam of. In thomson's plum pudding model of the atom, the electrons were embedded in a uniform sphere of positive. The electrons were the negative plums embedded in a positive. After discovering the electron in 1897, j j thomson proposed that the atom looked like a. Plum pudding is an. What Is The Plum Pudding Experiment.