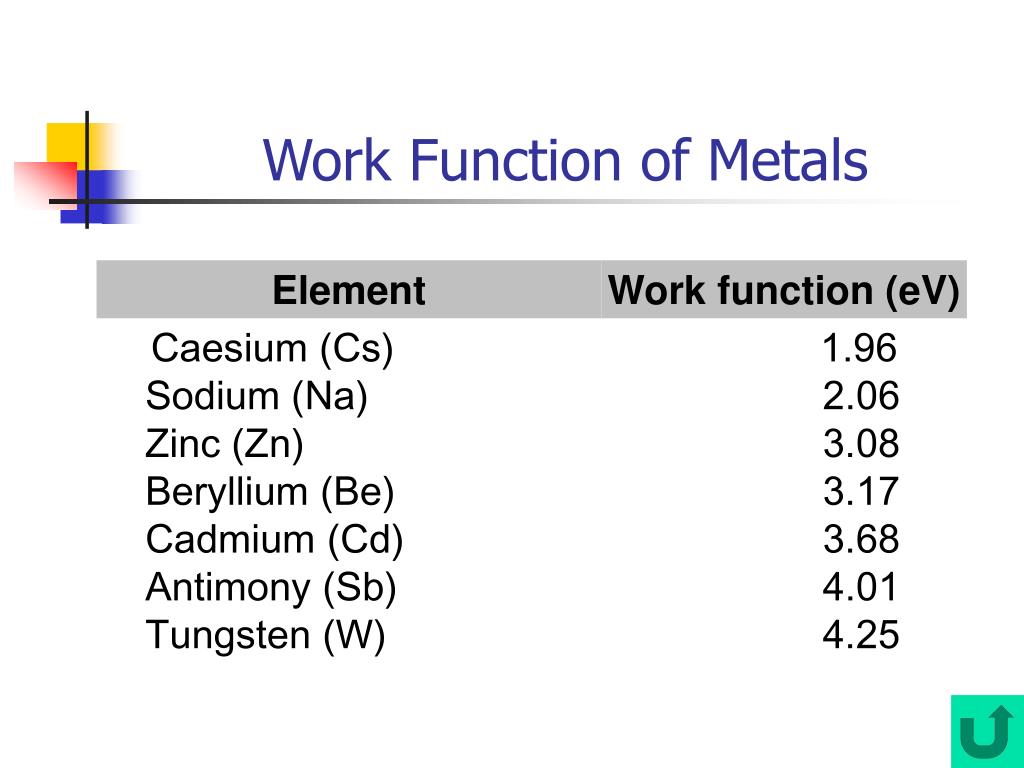
What Is The Work Function Of The Metal If The Light . If a radiation of wavelength 3000 ˚ a is incident on it, the maximum kinetic energy of the emitted. The work function of a metal is 2 e v. Alkali metals, such as sodium and potassium, have threshold frequencies in the visible light region. The photoelectric effect refers to the phenomenon where light, typically in the form of photons, can cause the emission of electrons from a material’s. Wavelength of light radiation required to just initiate photo emission from a material is 4000å, calculate the work function. This is because the attractive forces between the surface electrons and positive. What is the work function of the metal if a light of wavelength 4000 ∘a generates photoelectrons of velocity 6 ×105 ms−1 from it? The minimum energy required to remove an electron from a metal is called the work function, \ (w\) these are related by this equation:
from www.slideserve.com
Alkali metals, such as sodium and potassium, have threshold frequencies in the visible light region. The minimum energy required to remove an electron from a metal is called the work function, \ (w\) these are related by this equation: The work function of a metal is 2 e v. What is the work function of the metal if a light of wavelength 4000 ∘a generates photoelectrons of velocity 6 ×105 ms−1 from it? The photoelectric effect refers to the phenomenon where light, typically in the form of photons, can cause the emission of electrons from a material’s. This is because the attractive forces between the surface electrons and positive. If a radiation of wavelength 3000 ˚ a is incident on it, the maximum kinetic energy of the emitted. Wavelength of light radiation required to just initiate photo emission from a material is 4000å, calculate the work function.
PPT Electrons Inside The Atom PowerPoint Presentation, free download
What Is The Work Function Of The Metal If The Light The minimum energy required to remove an electron from a metal is called the work function, \ (w\) these are related by this equation: What is the work function of the metal if a light of wavelength 4000 ∘a generates photoelectrons of velocity 6 ×105 ms−1 from it? If a radiation of wavelength 3000 ˚ a is incident on it, the maximum kinetic energy of the emitted. The minimum energy required to remove an electron from a metal is called the work function, \ (w\) these are related by this equation: The work function of a metal is 2 e v. Alkali metals, such as sodium and potassium, have threshold frequencies in the visible light region. The photoelectric effect refers to the phenomenon where light, typically in the form of photons, can cause the emission of electrons from a material’s. This is because the attractive forces between the surface electrons and positive. Wavelength of light radiation required to just initiate photo emission from a material is 4000å, calculate the work function.
From www.vrogue.co
The Work Function Of A Metal Is 1 84 Ev If The Wavele vrogue.co What Is The Work Function Of The Metal If The Light What is the work function of the metal if a light of wavelength 4000 ∘a generates photoelectrons of velocity 6 ×105 ms−1 from it? The photoelectric effect refers to the phenomenon where light, typically in the form of photons, can cause the emission of electrons from a material’s. The work function of a metal is 2 e v. If a. What Is The Work Function Of The Metal If The Light.
From www.chegg.com
Solved The work functions for several metals are shown in What Is The Work Function Of The Metal If The Light The photoelectric effect refers to the phenomenon where light, typically in the form of photons, can cause the emission of electrons from a material’s. Wavelength of light radiation required to just initiate photo emission from a material is 4000å, calculate the work function. The minimum energy required to remove an electron from a metal is called the work function, \. What Is The Work Function Of The Metal If The Light.
From www.toppr.com
The work function for a metal is 40 eV. To emit photo electrons of zero What Is The Work Function Of The Metal If The Light Alkali metals, such as sodium and potassium, have threshold frequencies in the visible light region. Wavelength of light radiation required to just initiate photo emission from a material is 4000å, calculate the work function. What is the work function of the metal if a light of wavelength 4000 ∘a generates photoelectrons of velocity 6 ×105 ms−1 from it? This is. What Is The Work Function Of The Metal If The Light.
From www.youtube.com
The threshold wavelength for emission of electrons from a given metal What Is The Work Function Of The Metal If The Light The work function of a metal is 2 e v. The photoelectric effect refers to the phenomenon where light, typically in the form of photons, can cause the emission of electrons from a material’s. The minimum energy required to remove an electron from a metal is called the work function, \ (w\) these are related by this equation: If a. What Is The Work Function Of The Metal If The Light.
From www.youtube.com
The work function `(phi)` of some metals is listed below . The number What Is The Work Function Of The Metal If The Light The photoelectric effect refers to the phenomenon where light, typically in the form of photons, can cause the emission of electrons from a material’s. The work function of a metal is 2 e v. What is the work function of the metal if a light of wavelength 4000 ∘a generates photoelectrons of velocity 6 ×105 ms−1 from it? If a. What Is The Work Function Of The Metal If The Light.
From www.electrical4u.com
Work Function Formula & Relation To Threshold Frequency Electrical4U What Is The Work Function Of The Metal If The Light This is because the attractive forces between the surface electrons and positive. What is the work function of the metal if a light of wavelength 4000 ∘a generates photoelectrons of velocity 6 ×105 ms−1 from it? Wavelength of light radiation required to just initiate photo emission from a material is 4000å, calculate the work function. The work function of a. What Is The Work Function Of The Metal If The Light.
From www.chegg.com
Solved The work functions for several metals are shown in What Is The Work Function Of The Metal If The Light The minimum energy required to remove an electron from a metal is called the work function, \ (w\) these are related by this equation: Wavelength of light radiation required to just initiate photo emission from a material is 4000å, calculate the work function. This is because the attractive forces between the surface electrons and positive. If a radiation of wavelength. What Is The Work Function Of The Metal If The Light.
From byjus.com
The work function for Ag metal is 7.5×10^ 19 J. Ag metal is being What Is The Work Function Of The Metal If The Light The minimum energy required to remove an electron from a metal is called the work function, \ (w\) these are related by this equation: The work function of a metal is 2 e v. If a radiation of wavelength 3000 ˚ a is incident on it, the maximum kinetic energy of the emitted. The photoelectric effect refers to the phenomenon. What Is The Work Function Of The Metal If The Light.
From general.chemistrysteps.com
Photoelectric Effect Chemistry Steps What Is The Work Function Of The Metal If The Light The photoelectric effect refers to the phenomenon where light, typically in the form of photons, can cause the emission of electrons from a material’s. This is because the attractive forces between the surface electrons and positive. What is the work function of the metal if a light of wavelength 4000 ∘a generates photoelectrons of velocity 6 ×105 ms−1 from it?. What Is The Work Function Of The Metal If The Light.
From www.teachoo.com
[Term 2] Light of wavelength 2000 Å falls on a metal surface of work What Is The Work Function Of The Metal If The Light The photoelectric effect refers to the phenomenon where light, typically in the form of photons, can cause the emission of electrons from a material’s. Wavelength of light radiation required to just initiate photo emission from a material is 4000å, calculate the work function. The work function of a metal is 2 e v. What is the work function of the. What Is The Work Function Of The Metal If The Light.
From www.numerade.com
SOLVED 'The work function f magnasium metall is 5.86 1019 What Is The Work Function Of The Metal If The Light Alkali metals, such as sodium and potassium, have threshold frequencies in the visible light region. Wavelength of light radiation required to just initiate photo emission from a material is 4000å, calculate the work function. The photoelectric effect refers to the phenomenon where light, typically in the form of photons, can cause the emission of electrons from a material’s. The minimum. What Is The Work Function Of The Metal If The Light.
From www.toppr.com
The work function (ϕ) of some metals are listed below. The number of What Is The Work Function Of The Metal If The Light The minimum energy required to remove an electron from a metal is called the work function, \ (w\) these are related by this equation: The work function of a metal is 2 e v. What is the work function of the metal if a light of wavelength 4000 ∘a generates photoelectrons of velocity 6 ×105 ms−1 from it? Alkali metals,. What Is The Work Function Of The Metal If The Light.
From www.youtube.com
Light of wavelength 2000 Å falls on a metal surface of work function 4. What Is The Work Function Of The Metal If The Light Alkali metals, such as sodium and potassium, have threshold frequencies in the visible light region. What is the work function of the metal if a light of wavelength 4000 ∘a generates photoelectrons of velocity 6 ×105 ms−1 from it? If a radiation of wavelength 3000 ˚ a is incident on it, the maximum kinetic energy of the emitted. The minimum. What Is The Work Function Of The Metal If The Light.
From www.vrogue.co
The Work Function Of A Metal Is 1 84 Ev If The Wavele vrogue.co What Is The Work Function Of The Metal If The Light The minimum energy required to remove an electron from a metal is called the work function, \ (w\) these are related by this equation: If a radiation of wavelength 3000 ˚ a is incident on it, the maximum kinetic energy of the emitted. The photoelectric effect refers to the phenomenon where light, typically in the form of photons, can cause. What Is The Work Function Of The Metal If The Light.
From www.chegg.com
Solved The work function of copper metal is 437 kJ/mol. What What Is The Work Function Of The Metal If The Light This is because the attractive forces between the surface electrons and positive. The minimum energy required to remove an electron from a metal is called the work function, \ (w\) these are related by this equation: Alkali metals, such as sodium and potassium, have threshold frequencies in the visible light region. Wavelength of light radiation required to just initiate photo. What Is The Work Function Of The Metal If The Light.
From www.slideserve.com
PPT Electrons Inside The Atom PowerPoint Presentation, free download What Is The Work Function Of The Metal If The Light The photoelectric effect refers to the phenomenon where light, typically in the form of photons, can cause the emission of electrons from a material’s. Wavelength of light radiation required to just initiate photo emission from a material is 4000å, calculate the work function. The work function of a metal is 2 e v. This is because the attractive forces between. What Is The Work Function Of The Metal If The Light.
From www.toppr.com
When light of wavelength lesser than 6000 oA is incident on a metal What Is The Work Function Of The Metal If The Light What is the work function of the metal if a light of wavelength 4000 ∘a generates photoelectrons of velocity 6 ×105 ms−1 from it? The photoelectric effect refers to the phenomenon where light, typically in the form of photons, can cause the emission of electrons from a material’s. Wavelength of light radiation required to just initiate photo emission from a. What Is The Work Function Of The Metal If The Light.
From www.youtube.com
Work Function Problem YouTube What Is The Work Function Of The Metal If The Light What is the work function of the metal if a light of wavelength 4000 ∘a generates photoelectrons of velocity 6 ×105 ms−1 from it? This is because the attractive forces between the surface electrons and positive. The work function of a metal is 2 e v. Wavelength of light radiation required to just initiate photo emission from a material is. What Is The Work Function Of The Metal If The Light.
From byjus.com
The work function of caesium metal is 2.14 eV. When light of frequency What Is The Work Function Of The Metal If The Light The photoelectric effect refers to the phenomenon where light, typically in the form of photons, can cause the emission of electrons from a material’s. Wavelength of light radiation required to just initiate photo emission from a material is 4000å, calculate the work function. The work function of a metal is 2 e v. Alkali metals, such as sodium and potassium,. What Is The Work Function Of The Metal If The Light.
From www.chegg.com
Solved The work function of the metal in a photoelectric What Is The Work Function Of The Metal If The Light If a radiation of wavelength 3000 ˚ a is incident on it, the maximum kinetic energy of the emitted. The photoelectric effect refers to the phenomenon where light, typically in the form of photons, can cause the emission of electrons from a material’s. The minimum energy required to remove an electron from a metal is called the work function, \. What Is The Work Function Of The Metal If The Light.
From www.chegg.com
Solved A metal surface with a work function of 3.2x1019 J What Is The Work Function Of The Metal If The Light This is because the attractive forces between the surface electrons and positive. The minimum energy required to remove an electron from a metal is called the work function, \ (w\) these are related by this equation: What is the work function of the metal if a light of wavelength 4000 ∘a generates photoelectrons of velocity 6 ×105 ms−1 from it?. What Is The Work Function Of The Metal If The Light.
From www.chegg.com
Solved Light is incident on a metal that has a work function What Is The Work Function Of The Metal If The Light Alkali metals, such as sodium and potassium, have threshold frequencies in the visible light region. This is because the attractive forces between the surface electrons and positive. The minimum energy required to remove an electron from a metal is called the work function, \ (w\) these are related by this equation: If a radiation of wavelength 3000 ˚ a is. What Is The Work Function Of The Metal If The Light.
From www.chegg.com
Solved A certain metal having a work function of 5.4 eV is What Is The Work Function Of The Metal If The Light The minimum energy required to remove an electron from a metal is called the work function, \ (w\) these are related by this equation: The photoelectric effect refers to the phenomenon where light, typically in the form of photons, can cause the emission of electrons from a material’s. Alkali metals, such as sodium and potassium, have threshold frequencies in the. What Is The Work Function Of The Metal If The Light.
From www.researchgate.net
2 The work function and conductivity of metals. [161] Download What Is The Work Function Of The Metal If The Light Alkali metals, such as sodium and potassium, have threshold frequencies in the visible light region. The minimum energy required to remove an electron from a metal is called the work function, \ (w\) these are related by this equation: The work function of a metal is 2 e v. If a radiation of wavelength 3000 ˚ a is incident on. What Is The Work Function Of The Metal If The Light.
From www.vrogue.co
The Work Function Of A Metal Is 1 84 Ev If The Wavele vrogue.co What Is The Work Function Of The Metal If The Light The minimum energy required to remove an electron from a metal is called the work function, \ (w\) these are related by this equation: What is the work function of the metal if a light of wavelength 4000 ∘a generates photoelectrons of velocity 6 ×105 ms−1 from it? If a radiation of wavelength 3000 ˚ a is incident on it,. What Is The Work Function Of The Metal If The Light.
From www.toppr.com
The work function of a metal is l eV. On making light of wavelength What Is The Work Function Of The Metal If The Light The work function of a metal is 2 e v. The photoelectric effect refers to the phenomenon where light, typically in the form of photons, can cause the emission of electrons from a material’s. The minimum energy required to remove an electron from a metal is called the work function, \ (w\) these are related by this equation: Wavelength of. What Is The Work Function Of The Metal If The Light.
From byjus.com
The work function for Ag metal is 7.5×10^ 19J.Ag metal is beig exposed What Is The Work Function Of The Metal If The Light The photoelectric effect refers to the phenomenon where light, typically in the form of photons, can cause the emission of electrons from a material’s. If a radiation of wavelength 3000 ˚ a is incident on it, the maximum kinetic energy of the emitted. Wavelength of light radiation required to just initiate photo emission from a material is 4000å, calculate the. What Is The Work Function Of The Metal If The Light.
From www.numerade.com
SOLVED Two light sources are used in a photoelectric experiment to What Is The Work Function Of The Metal If The Light The work function of a metal is 2 e v. This is because the attractive forces between the surface electrons and positive. What is the work function of the metal if a light of wavelength 4000 ∘a generates photoelectrons of velocity 6 ×105 ms−1 from it? If a radiation of wavelength 3000 ˚ a is incident on it, the maximum. What Is The Work Function Of The Metal If The Light.
From www.chegg.com
Solved 4) In The Photoelectric Effect Experiment, The Gra... What Is The Work Function Of The Metal If The Light The photoelectric effect refers to the phenomenon where light, typically in the form of photons, can cause the emission of electrons from a material’s. What is the work function of the metal if a light of wavelength 4000 ∘a generates photoelectrons of velocity 6 ×105 ms−1 from it? This is because the attractive forces between the surface electrons and positive.. What Is The Work Function Of The Metal If The Light.
From www.slideshare.net
Photoelectric effect What Is The Work Function Of The Metal If The Light If a radiation of wavelength 3000 ˚ a is incident on it, the maximum kinetic energy of the emitted. The work function of a metal is 2 e v. The minimum energy required to remove an electron from a metal is called the work function, \ (w\) these are related by this equation: This is because the attractive forces between. What Is The Work Function Of The Metal If The Light.
From physicscalculations.com
How to Calculate the Work Function of a Metal What Is The Work Function Of The Metal If The Light The work function of a metal is 2 e v. The photoelectric effect refers to the phenomenon where light, typically in the form of photons, can cause the emission of electrons from a material’s. What is the work function of the metal if a light of wavelength 4000 ∘a generates photoelectrons of velocity 6 ×105 ms−1 from it? If a. What Is The Work Function Of The Metal If The Light.
From www.teachoo.com
[Term 2] Light of wavelength 2000 Å falls on a metal surface of work What Is The Work Function Of The Metal If The Light The work function of a metal is 2 e v. The photoelectric effect refers to the phenomenon where light, typically in the form of photons, can cause the emission of electrons from a material’s. Wavelength of light radiation required to just initiate photo emission from a material is 4000å, calculate the work function. This is because the attractive forces between. What Is The Work Function Of The Metal If The Light.
From www.youtube.com
Photoelectric Effect How to Calculate Work Function of a Metal YouTube What Is The Work Function Of The Metal If The Light The minimum energy required to remove an electron from a metal is called the work function, \ (w\) these are related by this equation: Alkali metals, such as sodium and potassium, have threshold frequencies in the visible light region. If a radiation of wavelength 3000 ˚ a is incident on it, the maximum kinetic energy of the emitted. Wavelength of. What Is The Work Function Of The Metal If The Light.
From www.slideserve.com
PPT Integrated Circuit Devices PowerPoint Presentation, free download What Is The Work Function Of The Metal If The Light The photoelectric effect refers to the phenomenon where light, typically in the form of photons, can cause the emission of electrons from a material’s. Alkali metals, such as sodium and potassium, have threshold frequencies in the visible light region. This is because the attractive forces between the surface electrons and positive. The minimum energy required to remove an electron from. What Is The Work Function Of The Metal If The Light.
From www.youtube.com
A photon of wavelength 4 X 107 m strikes on metal surface, work What Is The Work Function Of The Metal If The Light What is the work function of the metal if a light of wavelength 4000 ∘a generates photoelectrons of velocity 6 ×105 ms−1 from it? Wavelength of light radiation required to just initiate photo emission from a material is 4000å, calculate the work function. If a radiation of wavelength 3000 ˚ a is incident on it, the maximum kinetic energy of. What Is The Work Function Of The Metal If The Light.