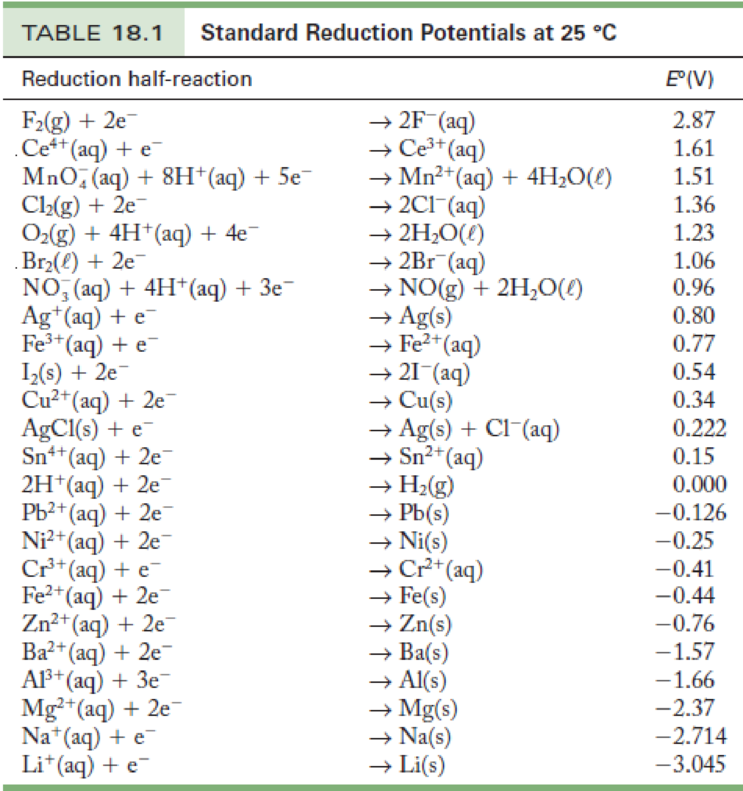
Standard Electrode Potential Numericals . Interpret electrode potentials in terms of relative oxidant and reductant strengths. Values relative to the standard hydrogen electrode (she). The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion combination. Wikipedia) t0 = 298.15 k (25.00°c) p0 =. 372 rows standard electrode potential (data page) the data below tabulates standard electrode potentials (e °), in volts relative to the standard. Describe and relate the definitions of electrode and cell potentials. The standard electrode potential is a physical quantity and, like all physical quantities, it is the product of a numerical value (a pure number) and.
from dinorahiu-images.blogspot.com
The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion combination. 372 rows standard electrode potential (data page) the data below tabulates standard electrode potentials (e °), in volts relative to the standard. Values relative to the standard hydrogen electrode (she). Interpret electrode potentials in terms of relative oxidant and reductant strengths. Describe and relate the definitions of electrode and cell potentials. Wikipedia) t0 = 298.15 k (25.00°c) p0 =. The standard electrode potential is a physical quantity and, like all physical quantities, it is the product of a numerical value (a pure number) and.
Standard Potential Table / 18 4 Standard Electrode Potential Powerpoint
Standard Electrode Potential Numericals Interpret electrode potentials in terms of relative oxidant and reductant strengths. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion combination. Values relative to the standard hydrogen electrode (she). The standard electrode potential is a physical quantity and, like all physical quantities, it is the product of a numerical value (a pure number) and. Describe and relate the definitions of electrode and cell potentials. Wikipedia) t0 = 298.15 k (25.00°c) p0 =. Interpret electrode potentials in terms of relative oxidant and reductant strengths. 372 rows standard electrode potential (data page) the data below tabulates standard electrode potentials (e °), in volts relative to the standard.
From pandai.me
Standard Electrode Potential Standard Electrode Potential Numericals Interpret electrode potentials in terms of relative oxidant and reductant strengths. Describe and relate the definitions of electrode and cell potentials. Wikipedia) t0 = 298.15 k (25.00°c) p0 =. The standard electrode potential is a physical quantity and, like all physical quantities, it is the product of a numerical value (a pure number) and. Values relative to the standard hydrogen. Standard Electrode Potential Numericals.
From slideplayer.com
ELECTROCHEMISTRY. ppt download Standard Electrode Potential Numericals 372 rows standard electrode potential (data page) the data below tabulates standard electrode potentials (e °), in volts relative to the standard. Interpret electrode potentials in terms of relative oxidant and reductant strengths. Values relative to the standard hydrogen electrode (she). The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions. Standard Electrode Potential Numericals.
From www.youtube.com
APSC132 lecture 5 03 Standard Electrode Potential YouTube Standard Electrode Potential Numericals Interpret electrode potentials in terms of relative oxidant and reductant strengths. Describe and relate the definitions of electrode and cell potentials. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion combination. The standard electrode potential is a. Standard Electrode Potential Numericals.
From www.nagwa.com
Question Video Calculating a Standard Cell Potential from Standard Standard Electrode Potential Numericals Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and reductant strengths. 372 rows standard electrode potential (data page) the data below tabulates standard electrode potentials (e °), in volts relative to the standard. The standard electrode potential is a physical quantity and, like all physical quantities, it is the product. Standard Electrode Potential Numericals.
From youtube.com
19.1.4 Predict whether a reaction will be spontaneous using standard Standard Electrode Potential Numericals The standard electrode potential is a physical quantity and, like all physical quantities, it is the product of a numerical value (a pure number) and. Describe and relate the definitions of electrode and cell potentials. Values relative to the standard hydrogen electrode (she). The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under. Standard Electrode Potential Numericals.
From byjus.com
Standard electrode potentials are Fe2+/Fe; E° = 0.44 volts Fe3+/Fe2+; E Standard Electrode Potential Numericals Interpret electrode potentials in terms of relative oxidant and reductant strengths. Describe and relate the definitions of electrode and cell potentials. The standard electrode potential is a physical quantity and, like all physical quantities, it is the product of a numerical value (a pure number) and. 372 rows standard electrode potential (data page) the data below tabulates standard electrode potentials. Standard Electrode Potential Numericals.
From cbsencertsolutiononline.blogspot.com
CBSE NCERT SOLUTIONS The standard electrode potentials at 298 K Standard Electrode Potential Numericals Interpret electrode potentials in terms of relative oxidant and reductant strengths. The standard electrode potential is a physical quantity and, like all physical quantities, it is the product of a numerical value (a pure number) and. Wikipedia) t0 = 298.15 k (25.00°c) p0 =. 372 rows standard electrode potential (data page) the data below tabulates standard electrode potentials (e °),. Standard Electrode Potential Numericals.
From www.youtube.com
Using Standard Electrode Potentials YouTube Standard Electrode Potential Numericals The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion combination. Describe and relate the definitions of electrode and cell potentials. The standard electrode potential is a physical quantity and, like all physical quantities, it is the product. Standard Electrode Potential Numericals.
From dinorahiu-images.blogspot.com
Standard Potential Table / 18 4 Standard Electrode Potential Powerpoint Standard Electrode Potential Numericals 372 rows standard electrode potential (data page) the data below tabulates standard electrode potentials (e °), in volts relative to the standard. Wikipedia) t0 = 298.15 k (25.00°c) p0 =. The standard electrode potential is a physical quantity and, like all physical quantities, it is the product of a numerical value (a pure number) and. Values relative to the standard. Standard Electrode Potential Numericals.
From mungfali.com
Standard Electrode Potential Table Standard Electrode Potential Numericals 372 rows standard electrode potential (data page) the data below tabulates standard electrode potentials (e °), in volts relative to the standard. Interpret electrode potentials in terms of relative oxidant and reductant strengths. Values relative to the standard hydrogen electrode (she). Wikipedia) t0 = 298.15 k (25.00°c) p0 =. The emf measured when a metal / metal ion electrode is. Standard Electrode Potential Numericals.
From www.alamy.com
Observed and calculated values for the standard electrode potentials Standard Electrode Potential Numericals The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion combination. Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and reductant strengths. The standard electrode potential is a. Standard Electrode Potential Numericals.
From users.highland.edu
Standard Potentials Standard Electrode Potential Numericals Values relative to the standard hydrogen electrode (she). Describe and relate the definitions of electrode and cell potentials. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion combination. Wikipedia) t0 = 298.15 k (25.00°c) p0 =. The. Standard Electrode Potential Numericals.
From saylordotorg.github.io
Standard Potentials Standard Electrode Potential Numericals Interpret electrode potentials in terms of relative oxidant and reductant strengths. 372 rows standard electrode potential (data page) the data below tabulates standard electrode potentials (e °), in volts relative to the standard. Wikipedia) t0 = 298.15 k (25.00°c) p0 =. Describe and relate the definitions of electrode and cell potentials. The emf measured when a metal / metal ion. Standard Electrode Potential Numericals.
From www.slideserve.com
PPT OxidationReduction Reactions PowerPoint Presentation, free Standard Electrode Potential Numericals Describe and relate the definitions of electrode and cell potentials. 372 rows standard electrode potential (data page) the data below tabulates standard electrode potentials (e °), in volts relative to the standard. Values relative to the standard hydrogen electrode (she). The standard electrode potential is a physical quantity and, like all physical quantities, it is the product of a numerical. Standard Electrode Potential Numericals.
From dxofmrhhh.blob.core.windows.net
Standard Electrode Potential Pdf at Arthur Baker blog Standard Electrode Potential Numericals Wikipedia) t0 = 298.15 k (25.00°c) p0 =. 372 rows standard electrode potential (data page) the data below tabulates standard electrode potentials (e °), in volts relative to the standard. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal /. Standard Electrode Potential Numericals.
From dxofmrhhh.blob.core.windows.net
Standard Electrode Potential Pdf at Arthur Baker blog Standard Electrode Potential Numericals Wikipedia) t0 = 298.15 k (25.00°c) p0 =. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion combination. The standard electrode potential is a physical quantity and, like all physical quantities, it is the product of a. Standard Electrode Potential Numericals.
From www.slideserve.com
PPT Chapter 20 PowerPoint Presentation, free download ID6976551 Standard Electrode Potential Numericals Values relative to the standard hydrogen electrode (she). The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion combination. Describe and relate the definitions of electrode and cell potentials. The standard electrode potential is a physical quantity and,. Standard Electrode Potential Numericals.
From saylordotorg.github.io
Standard Potentials Standard Electrode Potential Numericals The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion combination. Describe and relate the definitions of electrode and cell potentials. 372 rows standard electrode potential (data page) the data below tabulates standard electrode potentials (e °), in. Standard Electrode Potential Numericals.
From pandai.me
Standard Electrode Potential Standard Electrode Potential Numericals Values relative to the standard hydrogen electrode (she). 372 rows standard electrode potential (data page) the data below tabulates standard electrode potentials (e °), in volts relative to the standard. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal /. Standard Electrode Potential Numericals.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID4831905 Standard Electrode Potential Numericals Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and reductant strengths. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion combination. Wikipedia) t0 = 298.15 k (25.00°c). Standard Electrode Potential Numericals.
From www.slideserve.com
PPT Unit VII Redox & Electrochemistry PowerPoint Presentation ID Standard Electrode Potential Numericals Values relative to the standard hydrogen electrode (she). The standard electrode potential is a physical quantity and, like all physical quantities, it is the product of a numerical value (a pure number) and. Describe and relate the definitions of electrode and cell potentials. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under. Standard Electrode Potential Numericals.
From www.studocu.com
Standard Electrode Potentials Electrochemistry Studocu Standard Electrode Potential Numericals Describe and relate the definitions of electrode and cell potentials. Values relative to the standard hydrogen electrode (she). The standard electrode potential is a physical quantity and, like all physical quantities, it is the product of a numerical value (a pure number) and. Interpret electrode potentials in terms of relative oxidant and reductant strengths. The emf measured when a metal. Standard Electrode Potential Numericals.
From www.youtube.com
19.1 Standard electrode potential (HL) YouTube Standard Electrode Potential Numericals The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion combination. 372 rows standard electrode potential (data page) the data below tabulates standard electrode potentials (e °), in volts relative to the standard. The standard electrode potential is. Standard Electrode Potential Numericals.
From mungfali.com
Electrode Potential Series Standard Electrode Potential Numericals Values relative to the standard hydrogen electrode (she). Wikipedia) t0 = 298.15 k (25.00°c) p0 =. Interpret electrode potentials in terms of relative oxidant and reductant strengths. The standard electrode potential is a physical quantity and, like all physical quantities, it is the product of a numerical value (a pure number) and. 372 rows standard electrode potential (data page) the. Standard Electrode Potential Numericals.
From byjus.com
Detail explanation about positive and negative value of electrode Standard Electrode Potential Numericals Interpret electrode potentials in terms of relative oxidant and reductant strengths. Values relative to the standard hydrogen electrode (she). Describe and relate the definitions of electrode and cell potentials. The standard electrode potential is a physical quantity and, like all physical quantities, it is the product of a numerical value (a pure number) and. Wikipedia) t0 = 298.15 k (25.00°c). Standard Electrode Potential Numericals.
From www.vedantu.com
Oxidation Potential and Number Important Concepts and Tips for JEE Standard Electrode Potential Numericals Wikipedia) t0 = 298.15 k (25.00°c) p0 =. Values relative to the standard hydrogen electrode (she). Describe and relate the definitions of electrode and cell potentials. 372 rows standard electrode potential (data page) the data below tabulates standard electrode potentials (e °), in volts relative to the standard. The emf measured when a metal / metal ion electrode is coupled. Standard Electrode Potential Numericals.
From www.toppr.com
Using the standard electrode potentials given in the table, predict the Standard Electrode Potential Numericals 372 rows standard electrode potential (data page) the data below tabulates standard electrode potentials (e °), in volts relative to the standard. Interpret electrode potentials in terms of relative oxidant and reductant strengths. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of. Standard Electrode Potential Numericals.
From www.chegg.com
Solved 7.17 From the standard electrode potentials in Table Standard Electrode Potential Numericals 372 rows standard electrode potential (data page) the data below tabulates standard electrode potentials (e °), in volts relative to the standard. Interpret electrode potentials in terms of relative oxidant and reductant strengths. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of. Standard Electrode Potential Numericals.
From www.youtube.com
HOW WE MEASURE STANDARD ELECTRODE POTENTIAL VALUES YouTube Standard Electrode Potential Numericals Describe and relate the definitions of electrode and cell potentials. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion combination. Values relative to the standard hydrogen electrode (she). Interpret electrode potentials in terms of relative oxidant and. Standard Electrode Potential Numericals.
From byjus.com
6. The standard electrode potential E^° I2/I , E^° Br /Br2 and E^° Fe Standard Electrode Potential Numericals Describe and relate the definitions of electrode and cell potentials. Values relative to the standard hydrogen electrode (she). The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion combination. Interpret electrode potentials in terms of relative oxidant and. Standard Electrode Potential Numericals.
From question.pandai.org
Standard Electrode Potential Standard Electrode Potential Numericals Interpret electrode potentials in terms of relative oxidant and reductant strengths. Describe and relate the definitions of electrode and cell potentials. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion combination. Wikipedia) t0 = 298.15 k (25.00°c). Standard Electrode Potential Numericals.
From www.youtube.com
Measurement of electrode potential emf of cell electrochemistry Standard Electrode Potential Numericals Describe and relate the definitions of electrode and cell potentials. Values relative to the standard hydrogen electrode (she). The standard electrode potential is a physical quantity and, like all physical quantities, it is the product of a numerical value (a pure number) and. 372 rows standard electrode potential (data page) the data below tabulates standard electrode potentials (e °), in. Standard Electrode Potential Numericals.
From pt.scribd.com
Standard Electrode and Reduction Potentials at 298 K Printable Sets Standard Electrode Potential Numericals Describe and relate the definitions of electrode and cell potentials. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion combination. The standard electrode potential is a physical quantity and, like all physical quantities, it is the product. Standard Electrode Potential Numericals.
From testbook.com
Standard Electrode PotentialLearn Definition,Formula,Conditions Standard Electrode Potential Numericals The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion combination. Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and reductant strengths. The standard electrode potential is a. Standard Electrode Potential Numericals.
From www.flexiprep.com
Electrochemistry Electrode Potential and Standard Electrode Potential Standard Electrode Potential Numericals 372 rows standard electrode potential (data page) the data below tabulates standard electrode potentials (e °), in volts relative to the standard. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion combination. Describe and relate the definitions. Standard Electrode Potential Numericals.