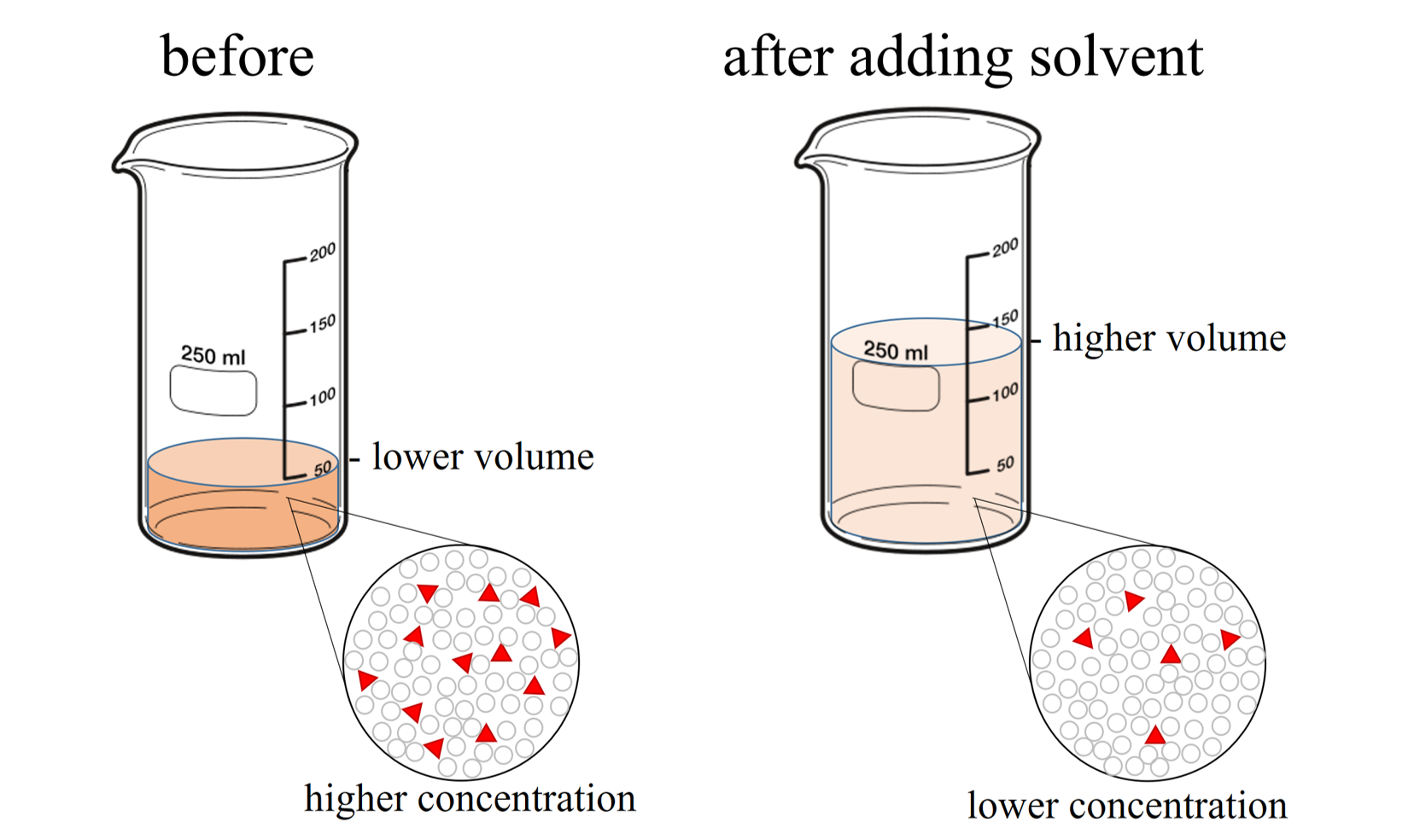
Anidulafungin Dilution Volume . Anidulafungin must be reconstituted with sterile water for injection and subsequently diluted with only 5% dextrose injection, usp or 0.9%. The compatibility of reconstituted anidulafungin with intravenous substances, additives, or medications other than 5% dextrose injection,. Dilution to a concentration of 0.77 mg/ml for the final infusion solution and infusion instructions for each dose. The rate of infusion should not exceed 1.1 mg/minute (equivalent to 1.4 ml/minute or 84 ml/hour when reconstituted and diluted per. The rate of infusion should not.
from chem.libretexts.org
The rate of infusion should not exceed 1.1 mg/minute (equivalent to 1.4 ml/minute or 84 ml/hour when reconstituted and diluted per. Dilution to a concentration of 0.77 mg/ml for the final infusion solution and infusion instructions for each dose. The compatibility of reconstituted anidulafungin with intravenous substances, additives, or medications other than 5% dextrose injection,. The rate of infusion should not. Anidulafungin must be reconstituted with sterile water for injection and subsequently diluted with only 5% dextrose injection, usp or 0.9%.
14.7 Solution Dilution Chemistry LibreTexts
Anidulafungin Dilution Volume Dilution to a concentration of 0.77 mg/ml for the final infusion solution and infusion instructions for each dose. The compatibility of reconstituted anidulafungin with intravenous substances, additives, or medications other than 5% dextrose injection,. Dilution to a concentration of 0.77 mg/ml for the final infusion solution and infusion instructions for each dose. The rate of infusion should not exceed 1.1 mg/minute (equivalent to 1.4 ml/minute or 84 ml/hour when reconstituted and diluted per. Anidulafungin must be reconstituted with sterile water for injection and subsequently diluted with only 5% dextrose injection, usp or 0.9%. The rate of infusion should not.
From www.researchgate.net
Rimonabant and anidulafungin inhibit ACE2spike binding. Enzymelinked... Download Scientific Anidulafungin Dilution Volume Anidulafungin must be reconstituted with sterile water for injection and subsequently diluted with only 5% dextrose injection, usp or 0.9%. The compatibility of reconstituted anidulafungin with intravenous substances, additives, or medications other than 5% dextrose injection,. Dilution to a concentration of 0.77 mg/ml for the final infusion solution and infusion instructions for each dose. The rate of infusion should not.. Anidulafungin Dilution Volume.
From www.researchgate.net
In vitro concentrationeffect relationship of anidulafungin against... Download Scientific Diagram Anidulafungin Dilution Volume The rate of infusion should not exceed 1.1 mg/minute (equivalent to 1.4 ml/minute or 84 ml/hour when reconstituted and diluted per. The rate of infusion should not. Anidulafungin must be reconstituted with sterile water for injection and subsequently diluted with only 5% dextrose injection, usp or 0.9%. Dilution to a concentration of 0.77 mg/ml for the final infusion solution and. Anidulafungin Dilution Volume.
From www.indiamart.com
Anidulafungin For Injection, 100mg 1 Vial, Treatment Severe Fungal Infections at Rs 10670/vial Anidulafungin Dilution Volume Anidulafungin must be reconstituted with sterile water for injection and subsequently diluted with only 5% dextrose injection, usp or 0.9%. The compatibility of reconstituted anidulafungin with intravenous substances, additives, or medications other than 5% dextrose injection,. Dilution to a concentration of 0.77 mg/ml for the final infusion solution and infusion instructions for each dose. The rate of infusion should not.. Anidulafungin Dilution Volume.
From www.researchgate.net
Dilution process of the sample. V a (ml) is aliquot volume and N,... Download Scientific Diagram Anidulafungin Dilution Volume The compatibility of reconstituted anidulafungin with intravenous substances, additives, or medications other than 5% dextrose injection,. Dilution to a concentration of 0.77 mg/ml for the final infusion solution and infusion instructions for each dose. The rate of infusion should not. The rate of infusion should not exceed 1.1 mg/minute (equivalent to 1.4 ml/minute or 84 ml/hour when reconstituted and diluted. Anidulafungin Dilution Volume.
From tajgenerics.com
Anidulafungin for Injection 100mg (FungsafeTAJ) Anidulafungin Dilution Volume The rate of infusion should not exceed 1.1 mg/minute (equivalent to 1.4 ml/minute or 84 ml/hour when reconstituted and diluted per. The rate of infusion should not. The compatibility of reconstituted anidulafungin with intravenous substances, additives, or medications other than 5% dextrose injection,. Anidulafungin must be reconstituted with sterile water for injection and subsequently diluted with only 5% dextrose injection,. Anidulafungin Dilution Volume.
From www.indiamart.com
Anidulafungin Aniducel 100mg injection, Treatment Severe Fungal Infections at Rs 5000/vial in Surat Anidulafungin Dilution Volume Anidulafungin must be reconstituted with sterile water for injection and subsequently diluted with only 5% dextrose injection, usp or 0.9%. The rate of infusion should not. The compatibility of reconstituted anidulafungin with intravenous substances, additives, or medications other than 5% dextrose injection,. Dilution to a concentration of 0.77 mg/ml for the final infusion solution and infusion instructions for each dose.. Anidulafungin Dilution Volume.
From www.indiamart.com
BDula 100 Anidulafungin Injection, 100mg Vial, Prescription at Rs 1400/piece in Dehradun Anidulafungin Dilution Volume The compatibility of reconstituted anidulafungin with intravenous substances, additives, or medications other than 5% dextrose injection,. The rate of infusion should not exceed 1.1 mg/minute (equivalent to 1.4 ml/minute or 84 ml/hour when reconstituted and diluted per. The rate of infusion should not. Anidulafungin must be reconstituted with sterile water for injection and subsequently diluted with only 5% dextrose injection,. Anidulafungin Dilution Volume.
From issuu.com
Anidulan (Anidulafungin) Uses, Side Effects and Precautions Aark Pharma by Aark Anidulafungin Dilution Volume The compatibility of reconstituted anidulafungin with intravenous substances, additives, or medications other than 5% dextrose injection,. The rate of infusion should not exceed 1.1 mg/minute (equivalent to 1.4 ml/minute or 84 ml/hour when reconstituted and diluted per. Dilution to a concentration of 0.77 mg/ml for the final infusion solution and infusion instructions for each dose. Anidulafungin must be reconstituted with. Anidulafungin Dilution Volume.
From www.researchgate.net
(a) Relationship between the anidulafungin C max /MIC ratio (using... Download Scientific Diagram Anidulafungin Dilution Volume Dilution to a concentration of 0.77 mg/ml for the final infusion solution and infusion instructions for each dose. Anidulafungin must be reconstituted with sterile water for injection and subsequently diluted with only 5% dextrose injection, usp or 0.9%. The rate of infusion should not. The compatibility of reconstituted anidulafungin with intravenous substances, additives, or medications other than 5% dextrose injection,.. Anidulafungin Dilution Volume.
From www.youtube.com
Chemistry 11 Dilution Calculations Solving for Initial Volume Example 3 YouTube Anidulafungin Dilution Volume The compatibility of reconstituted anidulafungin with intravenous substances, additives, or medications other than 5% dextrose injection,. The rate of infusion should not exceed 1.1 mg/minute (equivalent to 1.4 ml/minute or 84 ml/hour when reconstituted and diluted per. The rate of infusion should not. Dilution to a concentration of 0.77 mg/ml for the final infusion solution and infusion instructions for each. Anidulafungin Dilution Volume.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Anidulafungin Dilution Volume The rate of infusion should not. The rate of infusion should not exceed 1.1 mg/minute (equivalent to 1.4 ml/minute or 84 ml/hour when reconstituted and diluted per. Anidulafungin must be reconstituted with sterile water for injection and subsequently diluted with only 5% dextrose injection, usp or 0.9%. Dilution to a concentration of 0.77 mg/ml for the final infusion solution and. Anidulafungin Dilution Volume.
From www.researchgate.net
The inhibitory effect of anidulafungin on different stages of SFTSV... Download Scientific Diagram Anidulafungin Dilution Volume The compatibility of reconstituted anidulafungin with intravenous substances, additives, or medications other than 5% dextrose injection,. Dilution to a concentration of 0.77 mg/ml for the final infusion solution and infusion instructions for each dose. The rate of infusion should not. The rate of infusion should not exceed 1.1 mg/minute (equivalent to 1.4 ml/minute or 84 ml/hour when reconstituted and diluted. Anidulafungin Dilution Volume.
From www.researchgate.net
of anidulafungin in the plasma (raw data shown by... Download Scientific Diagram Anidulafungin Dilution Volume The rate of infusion should not exceed 1.1 mg/minute (equivalent to 1.4 ml/minute or 84 ml/hour when reconstituted and diluted per. The rate of infusion should not. The compatibility of reconstituted anidulafungin with intravenous substances, additives, or medications other than 5% dextrose injection,. Dilution to a concentration of 0.77 mg/ml for the final infusion solution and infusion instructions for each. Anidulafungin Dilution Volume.
From tajgenerics.com
Anidulafungin for injection 100 mg Drugs supplier Taj Generics Pharmaceuticals Taj Pharma Anidulafungin Dilution Volume Anidulafungin must be reconstituted with sterile water for injection and subsequently diluted with only 5% dextrose injection, usp or 0.9%. The rate of infusion should not. The compatibility of reconstituted anidulafungin with intravenous substances, additives, or medications other than 5% dextrose injection,. Dilution to a concentration of 0.77 mg/ml for the final infusion solution and infusion instructions for each dose.. Anidulafungin Dilution Volume.
From toku-e.com
AnidulafunginantifungalTOKUE Anidulafungin Dilution Volume Anidulafungin must be reconstituted with sterile water for injection and subsequently diluted with only 5% dextrose injection, usp or 0.9%. Dilution to a concentration of 0.77 mg/ml for the final infusion solution and infusion instructions for each dose. The compatibility of reconstituted anidulafungin with intravenous substances, additives, or medications other than 5% dextrose injection,. The rate of infusion should not.. Anidulafungin Dilution Volume.
From www.medicine.mcgill.ca
Serial Dilutions Anidulafungin Dilution Volume The compatibility of reconstituted anidulafungin with intravenous substances, additives, or medications other than 5% dextrose injection,. The rate of infusion should not exceed 1.1 mg/minute (equivalent to 1.4 ml/minute or 84 ml/hour when reconstituted and diluted per. The rate of infusion should not. Dilution to a concentration of 0.77 mg/ml for the final infusion solution and infusion instructions for each. Anidulafungin Dilution Volume.
From www.researchgate.net
Nonweightadjusted (a) and weightnormalized (b) anidulafungin... Download Scientific Diagram Anidulafungin Dilution Volume Dilution to a concentration of 0.77 mg/ml for the final infusion solution and infusion instructions for each dose. The compatibility of reconstituted anidulafungin with intravenous substances, additives, or medications other than 5% dextrose injection,. Anidulafungin must be reconstituted with sterile water for injection and subsequently diluted with only 5% dextrose injection, usp or 0.9%. The rate of infusion should not. Anidulafungin Dilution Volume.
From rowex.ie
Anidulafungin Rowex Consumer Healthcare Anidulafungin Dilution Volume Dilution to a concentration of 0.77 mg/ml for the final infusion solution and infusion instructions for each dose. The rate of infusion should not. The rate of infusion should not exceed 1.1 mg/minute (equivalent to 1.4 ml/minute or 84 ml/hour when reconstituted and diluted per. The compatibility of reconstituted anidulafungin with intravenous substances, additives, or medications other than 5% dextrose. Anidulafungin Dilution Volume.
From www.indiamart.com
Anidulafungin For Injection, 100 Mg/Vial, Treatment Severe Fungal Infections at Rs 10670/vial Anidulafungin Dilution Volume Dilution to a concentration of 0.77 mg/ml for the final infusion solution and infusion instructions for each dose. The rate of infusion should not exceed 1.1 mg/minute (equivalent to 1.4 ml/minute or 84 ml/hour when reconstituted and diluted per. The rate of infusion should not. The compatibility of reconstituted anidulafungin with intravenous substances, additives, or medications other than 5% dextrose. Anidulafungin Dilution Volume.
From www.indiamart.com
Anidulafungin 100mg Injection, Vial, Treatment Severe Fungal Infections at Rs 2000/piece in Surat Anidulafungin Dilution Volume Anidulafungin must be reconstituted with sterile water for injection and subsequently diluted with only 5% dextrose injection, usp or 0.9%. The rate of infusion should not. Dilution to a concentration of 0.77 mg/ml for the final infusion solution and infusion instructions for each dose. The rate of infusion should not exceed 1.1 mg/minute (equivalent to 1.4 ml/minute or 84 ml/hour. Anidulafungin Dilution Volume.
From www.researchgate.net
Antifungal susceptibility of planktonic and biofilm Candida determined... Download Table Anidulafungin Dilution Volume Dilution to a concentration of 0.77 mg/ml for the final infusion solution and infusion instructions for each dose. The compatibility of reconstituted anidulafungin with intravenous substances, additives, or medications other than 5% dextrose injection,. The rate of infusion should not. The rate of infusion should not exceed 1.1 mg/minute (equivalent to 1.4 ml/minute or 84 ml/hour when reconstituted and diluted. Anidulafungin Dilution Volume.
From www.alamy.com
Anidulafungin antifungal drug molecule. Skeletal formula Stock Photo Alamy Anidulafungin Dilution Volume The rate of infusion should not exceed 1.1 mg/minute (equivalent to 1.4 ml/minute or 84 ml/hour when reconstituted and diluted per. The compatibility of reconstituted anidulafungin with intravenous substances, additives, or medications other than 5% dextrose injection,. Dilution to a concentration of 0.77 mg/ml for the final infusion solution and infusion instructions for each dose. The rate of infusion should. Anidulafungin Dilution Volume.
From www.wikidoc.org
Anidulafungin wikidoc Anidulafungin Dilution Volume The rate of infusion should not. The rate of infusion should not exceed 1.1 mg/minute (equivalent to 1.4 ml/minute or 84 ml/hour when reconstituted and diluted per. Anidulafungin must be reconstituted with sterile water for injection and subsequently diluted with only 5% dextrose injection, usp or 0.9%. The compatibility of reconstituted anidulafungin with intravenous substances, additives, or medications other than. Anidulafungin Dilution Volume.
From www.ijidonline.com
of anidulafungin in critically ill patients with Candida peritonitis Anidulafungin Dilution Volume The rate of infusion should not exceed 1.1 mg/minute (equivalent to 1.4 ml/minute or 84 ml/hour when reconstituted and diluted per. The rate of infusion should not. Anidulafungin must be reconstituted with sterile water for injection and subsequently diluted with only 5% dextrose injection, usp or 0.9%. The compatibility of reconstituted anidulafungin with intravenous substances, additives, or medications other than. Anidulafungin Dilution Volume.
From www.slideserve.com
PPT Dilution Calculations PowerPoint Presentation, free download ID1709844 Anidulafungin Dilution Volume The rate of infusion should not exceed 1.1 mg/minute (equivalent to 1.4 ml/minute or 84 ml/hour when reconstituted and diluted per. The rate of infusion should not. Anidulafungin must be reconstituted with sterile water for injection and subsequently diluted with only 5% dextrose injection, usp or 0.9%. Dilution to a concentration of 0.77 mg/ml for the final infusion solution and. Anidulafungin Dilution Volume.
From www.alamy.com
Anidulafungin antifungal drug molecule. Skeletal formula Stock Vector Image & Art Alamy Anidulafungin Dilution Volume The compatibility of reconstituted anidulafungin with intravenous substances, additives, or medications other than 5% dextrose injection,. Dilution to a concentration of 0.77 mg/ml for the final infusion solution and infusion instructions for each dose. The rate of infusion should not exceed 1.1 mg/minute (equivalent to 1.4 ml/minute or 84 ml/hour when reconstituted and diluted per. The rate of infusion should. Anidulafungin Dilution Volume.
From tajgenerics.com
Anidulafungin for Injection 100mg (FungsafeTAJ) Anidulafungin Dilution Volume Anidulafungin must be reconstituted with sterile water for injection and subsequently diluted with only 5% dextrose injection, usp or 0.9%. The rate of infusion should not exceed 1.1 mg/minute (equivalent to 1.4 ml/minute or 84 ml/hour when reconstituted and diluted per. The compatibility of reconstituted anidulafungin with intravenous substances, additives, or medications other than 5% dextrose injection,. The rate of. Anidulafungin Dilution Volume.
From company.pharmahopers.com
Anidulafungin 50mg/100mg Injection Health Biotech Limited Anidulafungin Dilution Volume The rate of infusion should not. The compatibility of reconstituted anidulafungin with intravenous substances, additives, or medications other than 5% dextrose injection,. Dilution to a concentration of 0.77 mg/ml for the final infusion solution and infusion instructions for each dose. Anidulafungin must be reconstituted with sterile water for injection and subsequently diluted with only 5% dextrose injection, usp or 0.9%.. Anidulafungin Dilution Volume.
From www.indiamart.com
ANIDUBIO Allopathic Anidulafungin Injection, For Commercial, Prescription, ID 19790275688 Anidulafungin Dilution Volume The rate of infusion should not. The rate of infusion should not exceed 1.1 mg/minute (equivalent to 1.4 ml/minute or 84 ml/hour when reconstituted and diluted per. Anidulafungin must be reconstituted with sterile water for injection and subsequently diluted with only 5% dextrose injection, usp or 0.9%. Dilution to a concentration of 0.77 mg/ml for the final infusion solution and. Anidulafungin Dilution Volume.
From www.indiamart.com
Anidulafungin Injection (Canidula 100mg Injection ) at Rs 7500/piece Anidulafungin in Surat Anidulafungin Dilution Volume Dilution to a concentration of 0.77 mg/ml for the final infusion solution and infusion instructions for each dose. The rate of infusion should not. The rate of infusion should not exceed 1.1 mg/minute (equivalent to 1.4 ml/minute or 84 ml/hour when reconstituted and diluted per. Anidulafungin must be reconstituted with sterile water for injection and subsequently diluted with only 5%. Anidulafungin Dilution Volume.
From www.youtube.com
Chem 11 Dilution Calculations_Solving for Final Volume after Dilution Example 2 YouTube Anidulafungin Dilution Volume The rate of infusion should not. Dilution to a concentration of 0.77 mg/ml for the final infusion solution and infusion instructions for each dose. The rate of infusion should not exceed 1.1 mg/minute (equivalent to 1.4 ml/minute or 84 ml/hour when reconstituted and diluted per. The compatibility of reconstituted anidulafungin with intravenous substances, additives, or medications other than 5% dextrose. Anidulafungin Dilution Volume.
From www.carolina.com
Infographic—Lab Basics How to Perform Serial Dilutions Carolina Biological Supply Anidulafungin Dilution Volume The compatibility of reconstituted anidulafungin with intravenous substances, additives, or medications other than 5% dextrose injection,. The rate of infusion should not. The rate of infusion should not exceed 1.1 mg/minute (equivalent to 1.4 ml/minute or 84 ml/hour when reconstituted and diluted per. Dilution to a concentration of 0.77 mg/ml for the final infusion solution and infusion instructions for each. Anidulafungin Dilution Volume.
From www.researchgate.net
Pulmonary infiltrate volume determined by image analysis of serial CT... Download Scientific Anidulafungin Dilution Volume The compatibility of reconstituted anidulafungin with intravenous substances, additives, or medications other than 5% dextrose injection,. The rate of infusion should not exceed 1.1 mg/minute (equivalent to 1.4 ml/minute or 84 ml/hour when reconstituted and diluted per. The rate of infusion should not. Dilution to a concentration of 0.77 mg/ml for the final infusion solution and infusion instructions for each. Anidulafungin Dilution Volume.
From tajgenerics.com
Anidulafungin for Injection 100mg (FungsafeTAJ) Anidulafungin Dilution Volume Anidulafungin must be reconstituted with sterile water for injection and subsequently diluted with only 5% dextrose injection, usp or 0.9%. The compatibility of reconstituted anidulafungin with intravenous substances, additives, or medications other than 5% dextrose injection,. Dilution to a concentration of 0.77 mg/ml for the final infusion solution and infusion instructions for each dose. The rate of infusion should not.. Anidulafungin Dilution Volume.
From www.semanticscholar.org
Figure 1 from Anidulafungin for the treatment of candidaemia/invasive candidiasis in selected Anidulafungin Dilution Volume The rate of infusion should not. The rate of infusion should not exceed 1.1 mg/minute (equivalent to 1.4 ml/minute or 84 ml/hour when reconstituted and diluted per. Dilution to a concentration of 0.77 mg/ml for the final infusion solution and infusion instructions for each dose. Anidulafungin must be reconstituted with sterile water for injection and subsequently diluted with only 5%. Anidulafungin Dilution Volume.