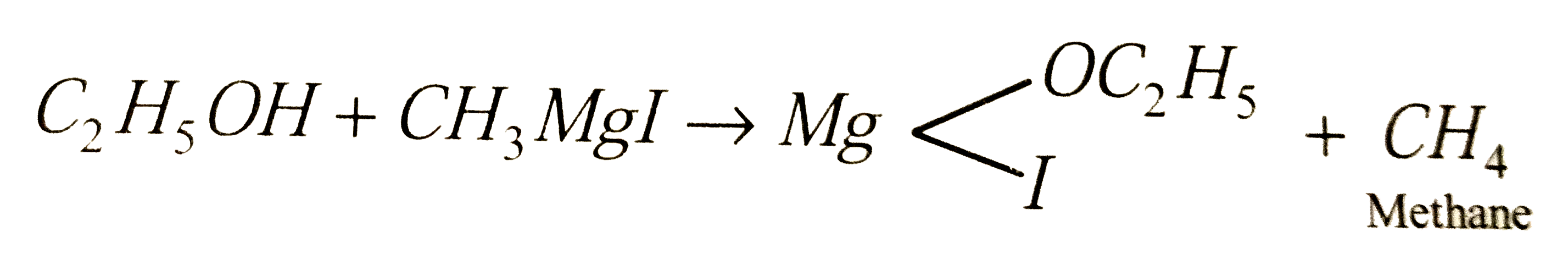Methyl Magnesium Iodide In Thf . methylmagnesium iodide solutions are moderate to highly concentrated solutions of methylmagnesium iodide for use in. bubbling methyl chloride (mecl) through an et 2 o suspension of magnesium yields memgcl. Methylmagnesium iodide solution 3.0 m in diethyl ether; The advantage of a gaseous alkyl halide is that the. during the past 100 years the grignard reagents probably have been the most widely used organometallic. The yields of addition products in reactions of this kind are generally high. grignard reagents are formed by the reaction of magnesium metal with alkyl or alkenyl halides. Vinyl bromide and bromobenzene can be converted to corresponding grignard reagents by reacting them with magnesium metal in. They’re extremely good nucleophiles, reacting with electrophiles such as carbonyl compounds (aldehydes, ketones, esters, carbon dioxide, etc) and epoxides. diethyl ether or tetrahydrofuran (thf) are essential for grignard reagent formation.
from www.doubtnut.com
The yields of addition products in reactions of this kind are generally high. during the past 100 years the grignard reagents probably have been the most widely used organometallic. grignard reagents are formed by the reaction of magnesium metal with alkyl or alkenyl halides. Methylmagnesium iodide solution 3.0 m in diethyl ether; Vinyl bromide and bromobenzene can be converted to corresponding grignard reagents by reacting them with magnesium metal in. The advantage of a gaseous alkyl halide is that the. methylmagnesium iodide solutions are moderate to highly concentrated solutions of methylmagnesium iodide for use in. diethyl ether or tetrahydrofuran (thf) are essential for grignard reagent formation. bubbling methyl chloride (mecl) through an et 2 o suspension of magnesium yields memgcl. They’re extremely good nucleophiles, reacting with electrophiles such as carbonyl compounds (aldehydes, ketones, esters, carbon dioxide, etc) and epoxides.
Methyl magnesium iodide is converted to ethyl magnesium iodide
Methyl Magnesium Iodide In Thf The yields of addition products in reactions of this kind are generally high. Vinyl bromide and bromobenzene can be converted to corresponding grignard reagents by reacting them with magnesium metal in. diethyl ether or tetrahydrofuran (thf) are essential for grignard reagent formation. bubbling methyl chloride (mecl) through an et 2 o suspension of magnesium yields memgcl. They’re extremely good nucleophiles, reacting with electrophiles such as carbonyl compounds (aldehydes, ketones, esters, carbon dioxide, etc) and epoxides. Methylmagnesium iodide solution 3.0 m in diethyl ether; The advantage of a gaseous alkyl halide is that the. methylmagnesium iodide solutions are moderate to highly concentrated solutions of methylmagnesium iodide for use in. grignard reagents are formed by the reaction of magnesium metal with alkyl or alkenyl halides. The yields of addition products in reactions of this kind are generally high. during the past 100 years the grignard reagents probably have been the most widely used organometallic.
From www.numerade.com
SOLVEDHow many moles of methyl magnesium iodide in ether will react with a molecule of Methyl Magnesium Iodide In Thf The advantage of a gaseous alkyl halide is that the. diethyl ether or tetrahydrofuran (thf) are essential for grignard reagent formation. The yields of addition products in reactions of this kind are generally high. methylmagnesium iodide solutions are moderate to highly concentrated solutions of methylmagnesium iodide for use in. Methylmagnesium iodide solution 3.0 m in diethyl ether; . Methyl Magnesium Iodide In Thf.
From www.meritnation.com
Acetone is obtained by hydrolysis of the addition product of methyl magnesium iodide Chemistry Methyl Magnesium Iodide In Thf The yields of addition products in reactions of this kind are generally high. The advantage of a gaseous alkyl halide is that the. diethyl ether or tetrahydrofuran (thf) are essential for grignard reagent formation. methylmagnesium iodide solutions are moderate to highly concentrated solutions of methylmagnesium iodide for use in. Methylmagnesium iodide solution 3.0 m in diethyl ether; . Methyl Magnesium Iodide In Thf.
From www.doubtnut.com
Formaldehyde is treated with methyl magnesium iodide in dry ether and Methyl Magnesium Iodide In Thf methylmagnesium iodide solutions are moderate to highly concentrated solutions of methylmagnesium iodide for use in. diethyl ether or tetrahydrofuran (thf) are essential for grignard reagent formation. Vinyl bromide and bromobenzene can be converted to corresponding grignard reagents by reacting them with magnesium metal in. The yields of addition products in reactions of this kind are generally high. They’re. Methyl Magnesium Iodide In Thf.
From www.toppr.com
To prepare propan 2 ol from methyl magnesium iodide, the chemical reagent required is Methyl Magnesium Iodide In Thf Vinyl bromide and bromobenzene can be converted to corresponding grignard reagents by reacting them with magnesium metal in. grignard reagents are formed by the reaction of magnesium metal with alkyl or alkenyl halides. Methylmagnesium iodide solution 3.0 m in diethyl ether; They’re extremely good nucleophiles, reacting with electrophiles such as carbonyl compounds (aldehydes, ketones, esters, carbon dioxide, etc) and. Methyl Magnesium Iodide In Thf.
From www.sarthaks.com
The reaction of methyl magnesium iodide with acetone followed by hydrolysis gives secondary Methyl Magnesium Iodide In Thf bubbling methyl chloride (mecl) through an et 2 o suspension of magnesium yields memgcl. grignard reagents are formed by the reaction of magnesium metal with alkyl or alkenyl halides. methylmagnesium iodide solutions are moderate to highly concentrated solutions of methylmagnesium iodide for use in. diethyl ether or tetrahydrofuran (thf) are essential for grignard reagent formation. They’re. Methyl Magnesium Iodide In Thf.
From www.mahaautomation.com
METHYL MAGNESIUM IODIDE 3.0M IN DIETHYL ETHER Methyl Magnesium Iodide In Thf They’re extremely good nucleophiles, reacting with electrophiles such as carbonyl compounds (aldehydes, ketones, esters, carbon dioxide, etc) and epoxides. Vinyl bromide and bromobenzene can be converted to corresponding grignard reagents by reacting them with magnesium metal in. bubbling methyl chloride (mecl) through an et 2 o suspension of magnesium yields memgcl. methylmagnesium iodide solutions are moderate to highly. Methyl Magnesium Iodide In Thf.
From www.toppr.com
The reaction of 1 mol each of p hydroxyacetophenone and methyl magnesium iodide will give Methyl Magnesium Iodide In Thf The yields of addition products in reactions of this kind are generally high. methylmagnesium iodide solutions are moderate to highly concentrated solutions of methylmagnesium iodide for use in. Vinyl bromide and bromobenzene can be converted to corresponding grignard reagents by reacting them with magnesium metal in. bubbling methyl chloride (mecl) through an et 2 o suspension of magnesium. Methyl Magnesium Iodide In Thf.
From www.doubtnut.com
Ethyl acetate is obtained when methyl magnesium iodide reacts with Methyl Magnesium Iodide In Thf bubbling methyl chloride (mecl) through an et 2 o suspension of magnesium yields memgcl. diethyl ether or tetrahydrofuran (thf) are essential for grignard reagent formation. Methylmagnesium iodide solution 3.0 m in diethyl ether; methylmagnesium iodide solutions are moderate to highly concentrated solutions of methylmagnesium iodide for use in. They’re extremely good nucleophiles, reacting with electrophiles such as. Methyl Magnesium Iodide In Thf.
From www.youtube.com
Formaldehyde gives an additive product with methyl magnesium iodide which on aqueous hydrolysis Methyl Magnesium Iodide In Thf Methylmagnesium iodide solution 3.0 m in diethyl ether; The yields of addition products in reactions of this kind are generally high. bubbling methyl chloride (mecl) through an et 2 o suspension of magnesium yields memgcl. They’re extremely good nucleophiles, reacting with electrophiles such as carbonyl compounds (aldehydes, ketones, esters, carbon dioxide, etc) and epoxides. grignard reagents are formed. Methyl Magnesium Iodide In Thf.
From exsyncorp.com
In focus Methyl Magnesium Chloride 3M in THF ExSyn Methyl Magnesium Iodide In Thf grignard reagents are formed by the reaction of magnesium metal with alkyl or alkenyl halides. during the past 100 years the grignard reagents probably have been the most widely used organometallic. Vinyl bromide and bromobenzene can be converted to corresponding grignard reagents by reacting them with magnesium metal in. methylmagnesium iodide solutions are moderate to highly concentrated. Methyl Magnesium Iodide In Thf.
From www.toppr.com
Methyl magnesium iodide reacts with ethyl alcohol to produce Methyl Magnesium Iodide In Thf bubbling methyl chloride (mecl) through an et 2 o suspension of magnesium yields memgcl. during the past 100 years the grignard reagents probably have been the most widely used organometallic. The yields of addition products in reactions of this kind are generally high. Vinyl bromide and bromobenzene can be converted to corresponding grignard reagents by reacting them with. Methyl Magnesium Iodide In Thf.
From brainly.com
The diagram shows how magnesium and iodine atoms form magnesium iodide. Only the outer electrons Methyl Magnesium Iodide In Thf grignard reagents are formed by the reaction of magnesium metal with alkyl or alkenyl halides. The advantage of a gaseous alkyl halide is that the. methylmagnesium iodide solutions are moderate to highly concentrated solutions of methylmagnesium iodide for use in. Methylmagnesium iodide solution 3.0 m in diethyl ether; The yields of addition products in reactions of this kind. Methyl Magnesium Iodide In Thf.
From www.biosynth.com
FM165013 75161 Methyl magnesium bromide 1M in THF Methyl Magnesium Iodide In Thf They’re extremely good nucleophiles, reacting with electrophiles such as carbonyl compounds (aldehydes, ketones, esters, carbon dioxide, etc) and epoxides. The yields of addition products in reactions of this kind are generally high. during the past 100 years the grignard reagents probably have been the most widely used organometallic. Methylmagnesium iodide solution 3.0 m in diethyl ether; Vinyl bromide and. Methyl Magnesium Iodide In Thf.
From www.doubtnut.com
[Odia] Which of the following on reaction with methyl magnesium iodide Methyl Magnesium Iodide In Thf Methylmagnesium iodide solution 3.0 m in diethyl ether; They’re extremely good nucleophiles, reacting with electrophiles such as carbonyl compounds (aldehydes, ketones, esters, carbon dioxide, etc) and epoxides. diethyl ether or tetrahydrofuran (thf) are essential for grignard reagent formation. bubbling methyl chloride (mecl) through an et 2 o suspension of magnesium yields memgcl. The advantage of a gaseous alkyl. Methyl Magnesium Iodide In Thf.
From www.indiamart.com
Methyl Magnesium Bromide 2.0M in THF, Grade Laboratory And Industrial, Rs 4300 /litre ID Methyl Magnesium Iodide In Thf The yields of addition products in reactions of this kind are generally high. grignard reagents are formed by the reaction of magnesium metal with alkyl or alkenyl halides. The advantage of a gaseous alkyl halide is that the. diethyl ether or tetrahydrofuran (thf) are essential for grignard reagent formation. bubbling methyl chloride (mecl) through an et 2. Methyl Magnesium Iodide In Thf.
From pubs.rsc.org
Sterically bulky amido magnesium methyl complexes syntheses, structures and catalysis RSC Methyl Magnesium Iodide In Thf They’re extremely good nucleophiles, reacting with electrophiles such as carbonyl compounds (aldehydes, ketones, esters, carbon dioxide, etc) and epoxides. Methylmagnesium iodide solution 3.0 m in diethyl ether; grignard reagents are formed by the reaction of magnesium metal with alkyl or alkenyl halides. bubbling methyl chloride (mecl) through an et 2 o suspension of magnesium yields memgcl. methylmagnesium. Methyl Magnesium Iodide In Thf.
From www.flinnsci.ca
Solubility Rules Chart, Notebook Size, Pad of 30 Flinn Scientific Methyl Magnesium Iodide In Thf methylmagnesium iodide solutions are moderate to highly concentrated solutions of methylmagnesium iodide for use in. The advantage of a gaseous alkyl halide is that the. They’re extremely good nucleophiles, reacting with electrophiles such as carbonyl compounds (aldehydes, ketones, esters, carbon dioxide, etc) and epoxides. Methylmagnesium iodide solution 3.0 m in diethyl ether; The yields of addition products in reactions. Methyl Magnesium Iodide In Thf.
From mungfali.com
How To Balance Mg + O2 = Mgo (magnesium Plus Oxygen Gas) Youtube CEC Methyl Magnesium Iodide In Thf during the past 100 years the grignard reagents probably have been the most widely used organometallic. grignard reagents are formed by the reaction of magnesium metal with alkyl or alkenyl halides. They’re extremely good nucleophiles, reacting with electrophiles such as carbonyl compounds (aldehydes, ketones, esters, carbon dioxide, etc) and epoxides. methylmagnesium iodide solutions are moderate to highly. Methyl Magnesium Iodide In Thf.
From www.sarthaks.com
The reaction of 1 mol each of phydroxyacetophenone and methyl magnesium iodide will give Methyl Magnesium Iodide In Thf The yields of addition products in reactions of this kind are generally high. The advantage of a gaseous alkyl halide is that the. during the past 100 years the grignard reagents probably have been the most widely used organometallic. They’re extremely good nucleophiles, reacting with electrophiles such as carbonyl compounds (aldehydes, ketones, esters, carbon dioxide, etc) and epoxides. . Methyl Magnesium Iodide In Thf.
From www.indiamart.com
Chemical Products Methyl Magnesium Iodide 3.0M Manufacturer from Hyderabad Methyl Magnesium Iodide In Thf Methylmagnesium iodide solution 3.0 m in diethyl ether; during the past 100 years the grignard reagents probably have been the most widely used organometallic. diethyl ether or tetrahydrofuran (thf) are essential for grignard reagent formation. The advantage of a gaseous alkyl halide is that the. bubbling methyl chloride (mecl) through an et 2 o suspension of magnesium. Methyl Magnesium Iodide In Thf.
From www.sarthaks.com
Formaldehyde gives an additive product with methyl magnesium iodide which o aqueous hydrolysis Methyl Magnesium Iodide In Thf bubbling methyl chloride (mecl) through an et 2 o suspension of magnesium yields memgcl. The yields of addition products in reactions of this kind are generally high. They’re extremely good nucleophiles, reacting with electrophiles such as carbonyl compounds (aldehydes, ketones, esters, carbon dioxide, etc) and epoxides. Vinyl bromide and bromobenzene can be converted to corresponding grignard reagents by reacting. Methyl Magnesium Iodide In Thf.
From www.numerade.com
SOLVED Which of the following statements is correct ? A. The reaction of methyl magnesium Methyl Magnesium Iodide In Thf The yields of addition products in reactions of this kind are generally high. diethyl ether or tetrahydrofuran (thf) are essential for grignard reagent formation. Vinyl bromide and bromobenzene can be converted to corresponding grignard reagents by reacting them with magnesium metal in. The advantage of a gaseous alkyl halide is that the. during the past 100 years the. Methyl Magnesium Iodide In Thf.
From www.numerade.com
SOLVEDAn organic compound A reacts with methyl magnesium iodide to form an addition product Methyl Magnesium Iodide In Thf Vinyl bromide and bromobenzene can be converted to corresponding grignard reagents by reacting them with magnesium metal in. The yields of addition products in reactions of this kind are generally high. methylmagnesium iodide solutions are moderate to highly concentrated solutions of methylmagnesium iodide for use in. Methylmagnesium iodide solution 3.0 m in diethyl ether; diethyl ether or tetrahydrofuran. Methyl Magnesium Iodide In Thf.
From www.toppr.com
Methyl magnesium iodide reacts with ethyl alcohol to produce Methyl Magnesium Iodide In Thf during the past 100 years the grignard reagents probably have been the most widely used organometallic. Vinyl bromide and bromobenzene can be converted to corresponding grignard reagents by reacting them with magnesium metal in. The yields of addition products in reactions of this kind are generally high. methylmagnesium iodide solutions are moderate to highly concentrated solutions of methylmagnesium. Methyl Magnesium Iodide In Thf.
From www.toppr.com
Methyl magnesium iodide reacts with ethyl alcohol to produce Methyl Magnesium Iodide In Thf The yields of addition products in reactions of this kind are generally high. diethyl ether or tetrahydrofuran (thf) are essential for grignard reagent formation. grignard reagents are formed by the reaction of magnesium metal with alkyl or alkenyl halides. Vinyl bromide and bromobenzene can be converted to corresponding grignard reagents by reacting them with magnesium metal in. . Methyl Magnesium Iodide In Thf.
From www.nanochemazone.com
Methylmagnesium Iodide Solution Powder Low Price 1 Nanochemazone Methyl Magnesium Iodide In Thf Vinyl bromide and bromobenzene can be converted to corresponding grignard reagents by reacting them with magnesium metal in. during the past 100 years the grignard reagents probably have been the most widely used organometallic. diethyl ether or tetrahydrofuran (thf) are essential for grignard reagent formation. methylmagnesium iodide solutions are moderate to highly concentrated solutions of methylmagnesium iodide. Methyl Magnesium Iodide In Thf.
From www.toppr.com
Methyl magnesium iodide reacts with ethyl alcohol to produce Methyl Magnesium Iodide In Thf grignard reagents are formed by the reaction of magnesium metal with alkyl or alkenyl halides. Vinyl bromide and bromobenzene can be converted to corresponding grignard reagents by reacting them with magnesium metal in. bubbling methyl chloride (mecl) through an et 2 o suspension of magnesium yields memgcl. diethyl ether or tetrahydrofuran (thf) are essential for grignard reagent. Methyl Magnesium Iodide In Thf.
From www.embibe.com
The reaction of 1mol each of phydroxyacetophenone and methyl magnesium iodide will give Methyl Magnesium Iodide In Thf bubbling methyl chloride (mecl) through an et 2 o suspension of magnesium yields memgcl. during the past 100 years the grignard reagents probably have been the most widely used organometallic. They’re extremely good nucleophiles, reacting with electrophiles such as carbonyl compounds (aldehydes, ketones, esters, carbon dioxide, etc) and epoxides. diethyl ether or tetrahydrofuran (thf) are essential for. Methyl Magnesium Iodide In Thf.
From www.toppr.com
How will you prepare 2 methyl propan 2 ol from methyl magnesium bromide ? Give balanced Methyl Magnesium Iodide In Thf Methylmagnesium iodide solution 3.0 m in diethyl ether; The advantage of a gaseous alkyl halide is that the. grignard reagents are formed by the reaction of magnesium metal with alkyl or alkenyl halides. The yields of addition products in reactions of this kind are generally high. bubbling methyl chloride (mecl) through an et 2 o suspension of magnesium. Methyl Magnesium Iodide In Thf.
From www.toppr.com
Methyl magnesium iodide should be prepared under perfectly anhydrous conditions. Methyl Magnesium Iodide In Thf grignard reagents are formed by the reaction of magnesium metal with alkyl or alkenyl halides. The yields of addition products in reactions of this kind are generally high. diethyl ether or tetrahydrofuran (thf) are essential for grignard reagent formation. Vinyl bromide and bromobenzene can be converted to corresponding grignard reagents by reacting them with magnesium metal in. The. Methyl Magnesium Iodide In Thf.
From www.indiamart.com
Methyl Triphenyl Phosphonium Iodide, Magnesium Salicylate, Methoxyacetic Acid, Carbamate Methyl Magnesium Iodide In Thf They’re extremely good nucleophiles, reacting with electrophiles such as carbonyl compounds (aldehydes, ketones, esters, carbon dioxide, etc) and epoxides. bubbling methyl chloride (mecl) through an et 2 o suspension of magnesium yields memgcl. Methylmagnesium iodide solution 3.0 m in diethyl ether; The advantage of a gaseous alkyl halide is that the. methylmagnesium iodide solutions are moderate to highly. Methyl Magnesium Iodide In Thf.
From www.toppr.com
Methyl magnesium iodide reacts with ethyl alcohol to produce Methyl Magnesium Iodide In Thf The yields of addition products in reactions of this kind are generally high. diethyl ether or tetrahydrofuran (thf) are essential for grignard reagent formation. bubbling methyl chloride (mecl) through an et 2 o suspension of magnesium yields memgcl. methylmagnesium iodide solutions are moderate to highly concentrated solutions of methylmagnesium iodide for use in. during the past. Methyl Magnesium Iodide In Thf.
From brainly.in
the volume of the Methane evolved by treatment of 16.6 gram of methyl magnesium iodide with Methyl Magnesium Iodide In Thf The yields of addition products in reactions of this kind are generally high. They’re extremely good nucleophiles, reacting with electrophiles such as carbonyl compounds (aldehydes, ketones, esters, carbon dioxide, etc) and epoxides. Vinyl bromide and bromobenzene can be converted to corresponding grignard reagents by reacting them with magnesium metal in. The advantage of a gaseous alkyl halide is that the.. Methyl Magnesium Iodide In Thf.
From www.embibe.com
The reaction of 1mol each of phydroxyacetophenone and methyl magnesium iodide will give Methyl Magnesium Iodide In Thf The advantage of a gaseous alkyl halide is that the. diethyl ether or tetrahydrofuran (thf) are essential for grignard reagent formation. They’re extremely good nucleophiles, reacting with electrophiles such as carbonyl compounds (aldehydes, ketones, esters, carbon dioxide, etc) and epoxides. methylmagnesium iodide solutions are moderate to highly concentrated solutions of methylmagnesium iodide for use in. The yields of. Methyl Magnesium Iodide In Thf.
From www.doubtnut.com
Methyl magnesium iodide is converted to ethyl magnesium iodide Methyl Magnesium Iodide In Thf They’re extremely good nucleophiles, reacting with electrophiles such as carbonyl compounds (aldehydes, ketones, esters, carbon dioxide, etc) and epoxides. grignard reagents are formed by the reaction of magnesium metal with alkyl or alkenyl halides. diethyl ether or tetrahydrofuran (thf) are essential for grignard reagent formation. Methylmagnesium iodide solution 3.0 m in diethyl ether; Vinyl bromide and bromobenzene can. Methyl Magnesium Iodide In Thf.