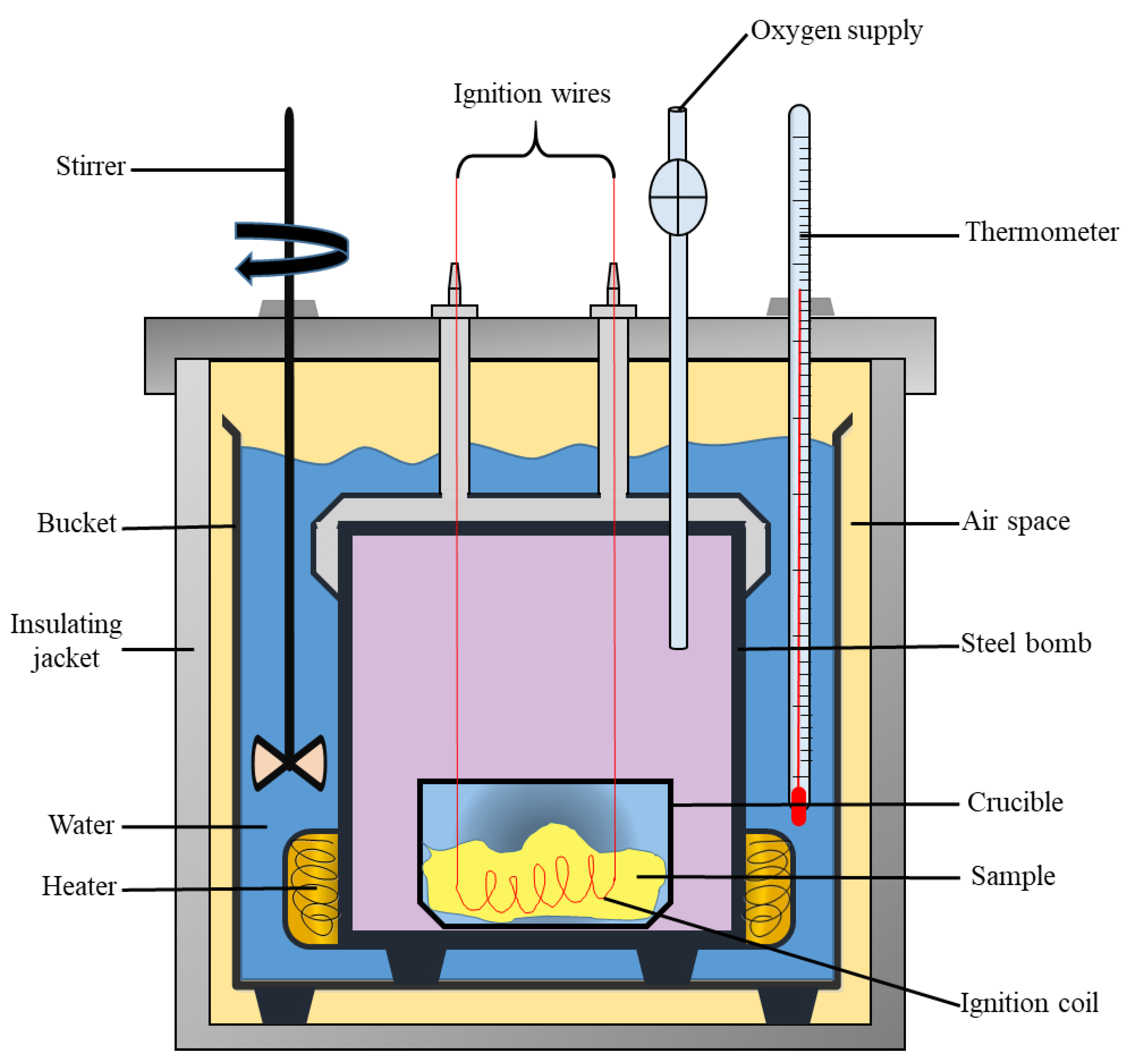
What Is Calorimetry Example . Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. the act or science of measuring the changes in the state variables of a body in order to calculate the heat. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. For example, when an exothermic reaction.
from www.animalia-life.club
calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. the act or science of measuring the changes in the state variables of a body in order to calculate the heat. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction.
Calorimeter Diagram
What Is Calorimetry Example calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. the act or science of measuring the changes in the state variables of a body in order to calculate the heat. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. For example, when an exothermic reaction. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction.
From www.animalia-life.club
Calorimeter Diagram What Is Calorimetry Example For example, when an exothermic reaction. the act or science of measuring the changes in the state variables of a body in order to calculate the heat. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature. What Is Calorimetry Example.
From www.youtube.com
Calorimetry calculation YouTube What Is Calorimetry Example a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction. the act or science of measuring the changes in the state variables of. What Is Calorimetry Example.
From www.youtube.com
How to find calorimeter constant YouTube What Is Calorimetry Example a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. the. What Is Calorimetry Example.
From exotbfkyp.blob.core.windows.net
What Is Calorimetry And Who Would Be Interested In Performing It at What Is Calorimetry Example a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. For example, when an exothermic reaction. a calorimeter is a device used to measure the amount of heat involved in a. What Is Calorimetry Example.
From www.youtube.com
050 Calorimetry YouTube What Is Calorimetry Example a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is. What Is Calorimetry Example.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts What Is Calorimetry Example a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. the. What Is Calorimetry Example.
From study.com
Calorimetry Definition, Equation & Types Lesson What Is Calorimetry Example calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. the act or science of measuring the changes in the state variables of a body in order to calculate the heat.. What Is Calorimetry Example.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3850751 What Is Calorimetry Example a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. the act or science of. What Is Calorimetry Example.
From saylordotorg.github.io
Calorimetry What Is Calorimetry Example a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction. the act or science of measuring the changes in the state variables of a body in order to calculate the heat. a calorimeter is a device used to measure the amount of. What Is Calorimetry Example.
From materialschreiner.z19.web.core.windows.net
Calorimetry Sample Problems With Solutions What Is Calorimetry Example the act or science of measuring the changes in the state variables of a body in order to calculate the heat. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction.. What Is Calorimetry Example.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 What Is Calorimetry Example For example, when an exothermic reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the process of measuring the amount of heat released or absorbed. What Is Calorimetry Example.
From www.chegg.com
Solved The Model Bomb Calorimetry Motorized stirrer What Is Calorimetry Example Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. a calorimeter is a device used to. What Is Calorimetry Example.
From www.showme.com
4 steps for solving calorimetry problems Science, Chemistry ShowMe What Is Calorimetry Example a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. For example, when an exothermic reaction. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. . What Is Calorimetry Example.
From www.tessshebaylo.com
Equation For Calorimetry Tessshebaylo What Is Calorimetry Example calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. For example, when an exothermic reaction. the act or science of measuring the changes in the state variables of a body in order to calculate the heat. Calorimetry is a field of thermochemistry that measures the amount of heat involved in. What Is Calorimetry Example.
From www.thoughtco.com
Calorimeter Definition in Chemistry What Is Calorimetry Example a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. For example, when an exothermic reaction.. What Is Calorimetry Example.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记1.8.3 Calorimetry翰林国际教育 What Is Calorimetry Example calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. the act or science of measuring the changes in the state variables of a body in order to calculate the heat. calorimetry is the. What Is Calorimetry Example.
From www.tessshebaylo.com
Equation For Determining Calorimetry Tessshebaylo What Is Calorimetry Example calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction. Calorimetry. What Is Calorimetry Example.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 What Is Calorimetry Example a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. the act or science of measuring the changes in the state variables of a body in order to. What Is Calorimetry Example.
From studylib.net
Calorimetry What Is Calorimetry Example a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. the act or science of measuring the changes in the state variables of a body in order to calculate the. What Is Calorimetry Example.
From schoolbag.info
Figure 7.6. Diagram of a Bomb Calorimeter What Is Calorimetry Example calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. the act. What Is Calorimetry Example.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals What Is Calorimetry Example calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the process. What Is Calorimetry Example.
From www.youtube.com
Physics 9.09b Calorimetry Example 1 YouTube What Is Calorimetry Example a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. the act or science of. What Is Calorimetry Example.
From www.youtube.com
CHEMISTRY 101 Constant Pressure Calorimetry YouTube What Is Calorimetry Example Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. the act or science of measuring the changes in the state variables of a body in order to calculate the heat. . What Is Calorimetry Example.
From www.youtube.com
Principle of Calorimetry YouTube What Is Calorimetry Example calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. the act or science of measuring the changes in the state variables of a body in order to calculate the heat. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. For example, when an exothermic. What Is Calorimetry Example.
From www.pinterest.com
Calorimetry Lab Report Science diagrams, Thermodynamics, How to find out What Is Calorimetry Example Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter. What Is Calorimetry Example.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types What Is Calorimetry Example a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. a calorimeter is a device used. What Is Calorimetry Example.
From www.youtube.com
How To Solve Basic Calorimetry Problems in Chemistry YouTube What Is Calorimetry Example For example, when an exothermic reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature. What Is Calorimetry Example.
From exovxxbwq.blob.core.windows.net
Calorimeter In Chemical Engineering at Elizabeth Hodgson blog What Is Calorimetry Example For example, when an exothermic reaction. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. a. What Is Calorimetry Example.
From physique.ensc-rennes.fr
TP calorimetry What Is Calorimetry Example calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. For example, when an exothermic reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. the act or science of measuring the changes in the state variables of a body. What Is Calorimetry Example.
From www.youtube.com
Final Temperature Calorimetry Practice Problems Chemistry YouTube What Is Calorimetry Example a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. calorimetry is the process. What Is Calorimetry Example.
From www.youtube.com
Physics 9.09g Calorimetry Example 2 YouTube What Is Calorimetry Example For example, when an exothermic reaction. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. . What Is Calorimetry Example.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube What Is Calorimetry Example calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. For example, when. What Is Calorimetry Example.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry What Is Calorimetry Example calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. the act or science of measuring. What Is Calorimetry Example.
From saylordotorg.github.io
Calorimetry What Is Calorimetry Example calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. For example, when an exothermic reaction. the act or science of measuring the changes in the state variables of a body in order to calculate the. What Is Calorimetry Example.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube What Is Calorimetry Example a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. For example, when an exothermic reaction. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change.. What Is Calorimetry Example.