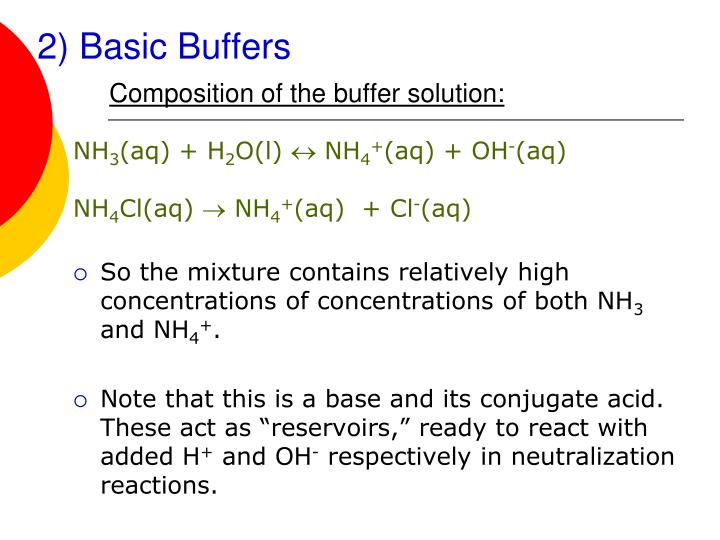
Common Basic Buffers . Either a weak acid plus a salt derived from that weak acid or a weak base plus a salt of that weak base. Calculate the ph of a buffer before and after the addition of added acid or. It is able to neutralize small amounts of added acid or base, thus. Buffers do so by being composed of certain pairs of solutes: A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called buffer solution. In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The mechanism involves a buffer, a solution that resists dramatic changes in ph.
from www.slideserve.com
A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The mechanism involves a buffer, a solution that resists dramatic changes in ph. Either a weak acid plus a salt derived from that weak acid or a weak base plus a salt of that weak base. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called buffer solution. Buffers do so by being composed of certain pairs of solutes: In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. Calculate the ph of a buffer before and after the addition of added acid or. It is able to neutralize small amounts of added acid or base, thus.
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint
Common Basic Buffers The mechanism involves a buffer, a solution that resists dramatic changes in ph. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called buffer solution. The mechanism involves a buffer, a solution that resists dramatic changes in ph. In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small amounts of added acid or base, thus. Either a weak acid plus a salt derived from that weak acid or a weak base plus a salt of that weak base. Calculate the ph of a buffer before and after the addition of added acid or. Buffers do so by being composed of certain pairs of solutes:
From chemistrypubs.com
Buffer Solution Definition, Examples, Mechanisms, Preparations Common Basic Buffers A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called buffer solution. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffers do so by being composed of certain pairs of solutes: Calculate the ph. Common Basic Buffers.
From www.youtube.com
2 Buffers Basic Buffers YouTube Common Basic Buffers Calculate the ph of a buffer before and after the addition of added acid or. Either a weak acid plus a salt derived from that weak acid or a weak base plus a salt of that weak base. It is able to neutralize small amounts of added acid or base, thus. A buffer is a solution that can resist ph. Common Basic Buffers.
From www.youtube.com
What is buffer types of buffer Acidic buffer and basic buffer Common Basic Buffers Calculate the ph of a buffer before and after the addition of added acid or. The mechanism involves a buffer, a solution that resists dramatic changes in ph. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called buffer solution. In chemistry, the definition of. Common Basic Buffers.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent Common Basic Buffers It is able to neutralize small amounts of added acid or base, thus. Either a weak acid plus a salt derived from that weak acid or a weak base plus a salt of that weak base. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffers do so by being. Common Basic Buffers.
From slidetodoc.com
Introduction to Buffers These solutions contain relatively high Common Basic Buffers A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called buffer solution. In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. Buffers do so by being composed of certain pairs. Common Basic Buffers.
From www.youtube.com
What is Buffer Solution Types of Buffer Solution Acidic Buffer Common Basic Buffers It is able to neutralize small amounts of added acid or base, thus. In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffers do so by. Common Basic Buffers.
From www.youtube.com
Chapter 14 part 16, additions to buffer and basic buffers YouTube Common Basic Buffers The mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. A solution whose ph. Common Basic Buffers.
From chemistrytalk.org
What is a Buffer Solution? Chemistry ChemTalk Common Basic Buffers A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called buffer solution. Calculate the ph of a buffer before and after the addition of added acid or. In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition. Common Basic Buffers.
From www.sliderbase.com
Buffers Presentation Chemistry Common Basic Buffers It is able to neutralize small amounts of added acid or base, thus. Calculate the ph of a buffer before and after the addition of added acid or. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called buffer solution. In chemistry, the definition of. Common Basic Buffers.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Common Basic Buffers It is able to neutralize small amounts of added acid or base, thus. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Calculate the ph of a buffer before and after the addition of added acid or. In chemistry, the definition of a buffer is a solution that can resist. Common Basic Buffers.
From www.youtube.com
buffer actions electrochemistry class 12 chemistry subject notes Common Basic Buffers Either a weak acid plus a salt derived from that weak acid or a weak base plus a salt of that weak base. It is able to neutralize small amounts of added acid or base, thus. In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base.. Common Basic Buffers.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID1031156 Common Basic Buffers Buffers do so by being composed of certain pairs of solutes: The mechanism involves a buffer, a solution that resists dramatic changes in ph. Calculate the ph of a buffer before and after the addition of added acid or. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid. Common Basic Buffers.
From exonxjimh.blob.core.windows.net
Common Buffers Used In Biochemistry at Betty Beckles blog Common Basic Buffers Either a weak acid plus a salt derived from that weak acid or a weak base plus a salt of that weak base. Buffers do so by being composed of certain pairs of solutes: A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. In chemistry, the definition of a buffer. Common Basic Buffers.
From www.slideserve.com
PPT Chapter 18 Other Aspects of Aqueous Equilibria PowerPoint Common Basic Buffers The mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. It is able to. Common Basic Buffers.
From magmastory.blogspot.com
Which Of The Following Represents A Buffer System? magmastory Common Basic Buffers Calculate the ph of a buffer before and after the addition of added acid or. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small amounts of added acid or base, thus. The mechanism involves a buffer, a solution that resists dramatic changes in ph.. Common Basic Buffers.
From design.udlvirtual.edu.pe
How Does A Circular Buffer Work Design Talk Common Basic Buffers A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Either a weak acid plus a salt derived from that weak acid or a weak base plus a salt of that weak base. A solution whose ph is not altered to any great extent by the addition of small quantities of. Common Basic Buffers.
From www.slideserve.com
PPT Chapter 18 Other Aspects of Aqueous Equilibria PowerPoint Common Basic Buffers A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffers do so by being composed of certain pairs of solutes: In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. It is able to neutralize small. Common Basic Buffers.
From slideplayer.com
SCH4U Acids and Bases BUFFER SOLUTIONS. ppt download Common Basic Buffers A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called buffer solution. Calculate the ph of a buffer before and after the addition of added acid. Common Basic Buffers.
From app.jove.com
Buffers, Buffer Components and Buffer Action Chemistry JoVe Common Basic Buffers It is able to neutralize small amounts of added acid or base, thus. Either a weak acid plus a salt derived from that weak acid or a weak base plus a salt of that weak base. In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base.. Common Basic Buffers.
From www.researchgate.net
List of common buffer components and their maximum allowed Common Basic Buffers The mechanism involves a buffer, a solution that resists dramatic changes in ph. Buffers do so by being composed of certain pairs of solutes: A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A solution whose ph is not altered to any great extent by the addition of small quantities. Common Basic Buffers.
From chem.libretexts.org
14.6 Buffers Chemistry LibreTexts Common Basic Buffers In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The mechanism involves a buffer, a solution that resists dramatic changes in ph. Either a weak acid. Common Basic Buffers.
From www.pinterest.es
Understanding Buffer Solution Chemistry A Complete Guide Common Basic Buffers A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called buffer solution. It is able to neutralize small amounts of added acid or base, thus. The mechanism involves a buffer, a solution that resists dramatic changes in ph. Calculate the ph of a buffer before. Common Basic Buffers.
From www.slideserve.com
PPT Buffers and Salts PowerPoint Presentation, free download ID5405242 Common Basic Buffers A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called buffer solution. The mechanism involves a buffer, a solution that resists dramatic changes in ph. In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an. Common Basic Buffers.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation Common Basic Buffers Buffers do so by being composed of certain pairs of solutes: It is able to neutralize small amounts of added acid or base, thus. Calculate the ph of a buffer before and after the addition of added acid or. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. In chemistry,. Common Basic Buffers.
From www.youtube.com
Acidic and Basic Buffers YouTube Common Basic Buffers A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is called buffer solution. The mechanism involves a buffer, a solution that resists dramatic changes in ph. In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an. Common Basic Buffers.
From www.youtube.com
Buffer Action Acidic and basic buffer Action how buffer resist pH Common Basic Buffers It is able to neutralize small amounts of added acid or base, thus. In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. Buffers do so by being composed of certain pairs of solutes: A buffer is a solution that can resist ph change upon the. Common Basic Buffers.
From www.slideshare.net
Buffers in chemical analysis, types of buffers Common Basic Buffers A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. The mechanism involves a buffer, a solution that resists dramatic changes in ph. It is able to. Common Basic Buffers.
From www.biobasic.com
Common Buffers Biological Buffers Biochemicals Products Common Basic Buffers It is able to neutralize small amounts of added acid or base, thus. In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Calculate the ph of. Common Basic Buffers.
From www.slideserve.com
PPT AcidBase Equilibria PowerPoint Presentation, free download ID Common Basic Buffers The mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Either a weak acid plus a salt derived from that weak acid or a weak base plus a salt of that weak base. It is able to neutralize. Common Basic Buffers.
From classnotes.org.in
Buffer solution and Buffer Action Chemistry, Class 11, Ionic Equilibrium Common Basic Buffers A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Calculate the ph of a buffer before and after the addition of added acid or. Buffers do so by being composed of certain pairs of solutes: A solution whose ph is not altered to any great extent by the addition of. Common Basic Buffers.
From www.slideserve.com
PPT Reversed Phase HPLC Mechanisms PowerPoint Presentation ID260343 Common Basic Buffers Calculate the ph of a buffer before and after the addition of added acid or. In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. It is able to neutralize small amounts of added acid or base, thus. Buffers do so by being composed of certain. Common Basic Buffers.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Common Basic Buffers Calculate the ph of a buffer before and after the addition of added acid or. It is able to neutralize small amounts of added acid or base, thus. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffers do so by being composed of certain pairs of solutes: The mechanism. Common Basic Buffers.
From 2012books.lardbucket.org
Buffers Common Basic Buffers The mechanism involves a buffer, a solution that resists dramatic changes in ph. Buffers do so by being composed of certain pairs of solutes: Calculate the ph of a buffer before and after the addition of added acid or. In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid. Common Basic Buffers.
From www.youtube.com
Acidic and Basic buffer Identification and Preparation Tips and Tricks Common Basic Buffers A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Either a weak acid plus a salt derived from that weak acid or a weak base plus a salt of that weak base. A solution whose ph is not altered to any great extent by the addition of small quantities of. Common Basic Buffers.
From id.pinterest.com
buffer solution , Acidic & basic buffer,weak acid +salt of weak acid Common Basic Buffers Either a weak acid plus a salt derived from that weak acid or a weak base plus a salt of that weak base. In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. Buffers do so by being composed of certain pairs of solutes: Calculate the. Common Basic Buffers.