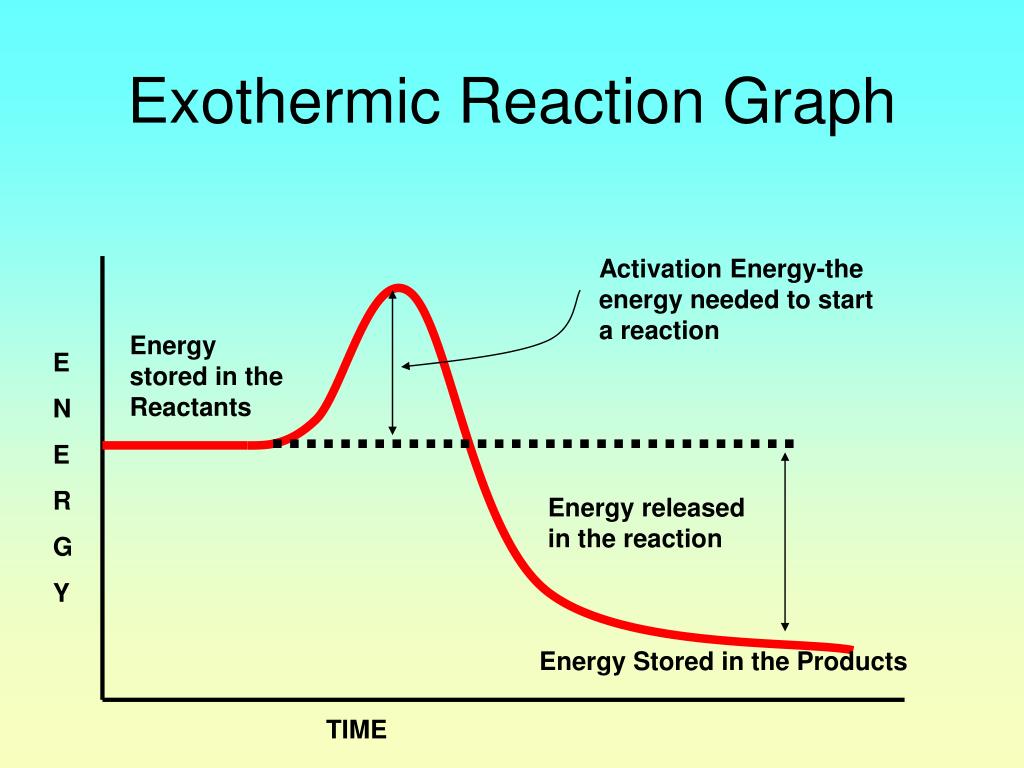
Endothermic Reaction Explanation . a chemical reaction is said to be endothermic when it absorbs energy, mostly heat. in endothermic reactions, more energy is absorbed when the bonds in the reactants are broken than is released when new bonds are. some examples of endothermic reactions are: In endothermic and exothermic reactions, energy can be thought of as either. exothermic and endothermic reactions. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. The reaction between ethanoic acid and sodium carbonate. The heat is absorbed from the surroundings allowing the reactants to transform into. revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from.
from www.slideserve.com
In endothermic and exothermic reactions, energy can be thought of as either. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. in endothermic reactions, more energy is absorbed when the bonds in the reactants are broken than is released when new bonds are. The heat is absorbed from the surroundings allowing the reactants to transform into. some examples of endothermic reactions are: revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. exothermic and endothermic reactions. a chemical reaction is said to be endothermic when it absorbs energy, mostly heat. The reaction between ethanoic acid and sodium carbonate.
PPT Endothermic Vs. Exothermic Reaction Graphs PowerPoint
Endothermic Reaction Explanation In endothermic and exothermic reactions, energy can be thought of as either. in endothermic reactions, more energy is absorbed when the bonds in the reactants are broken than is released when new bonds are. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. The heat is absorbed from the surroundings allowing the reactants to transform into. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. exothermic and endothermic reactions. The reaction between ethanoic acid and sodium carbonate. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. a chemical reaction is said to be endothermic when it absorbs energy, mostly heat. some examples of endothermic reactions are: In endothermic and exothermic reactions, energy can be thought of as either. revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from.
From www.aakash.ac.in
Endothermic Reactions in Chemistry Definition, Types and Importance of Endothermic Reaction Explanation The heat is absorbed from the surroundings allowing the reactants to transform into. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. In endothermic and exothermic reactions, energy can be thought of as either. some examples of endothermic reactions are: a chemical reaction is said to be endothermic when it absorbs energy, mostly. Endothermic Reaction Explanation.
From gamesmartz.com
Endothermic Reaction Easy to Understand Endothermic Reaction Explanation These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from. In endothermic and exothermic reactions, energy can be thought of as either. a chemical reaction is said to be endothermic when it absorbs energy,. Endothermic Reaction Explanation.
From www.slideserve.com
PPT Endothermic and Exothermic Reactions PowerPoint Presentation Endothermic Reaction Explanation These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. In endothermic and exothermic reactions, energy can be thought of as either. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. endothermic reactions are chemical reactions in which the reactants absorb heat energy from. Endothermic Reaction Explanation.
From materialmediabrindle.z21.web.core.windows.net
Endothermic Reactions And Exothermic Reaction Endothermic Reaction Explanation The heat is absorbed from the surroundings allowing the reactants to transform into. In endothermic and exothermic reactions, energy can be thought of as either. in endothermic reactions, more energy is absorbed when the bonds in the reactants are broken than is released when new bonds are. exothermic and endothermic reactions. revise and understand what endothermic and. Endothermic Reaction Explanation.
From byjus.com
Difference Between Endothermic and Exothermic Reactions Chemistry Endothermic Reaction Explanation These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. a chemical reaction is said to be endothermic when it absorbs energy, mostly heat. some examples of endothermic reactions are: The heat is absorbed. Endothermic Reaction Explanation.
From www.slideserve.com
PPT Endothermic & Exothermic Reactions PowerPoint Presentation ID Endothermic Reaction Explanation revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from. In endothermic and exothermic reactions, energy can be thought of as either. some examples of endothermic reactions are: endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. These. Endothermic Reaction Explanation.
From schematicunkenbz.z14.web.core.windows.net
Endothermic And Exothermic Reaction Diagram Endothermic Reaction Explanation exothermic and endothermic reactions. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. a chemical reaction is said to be endothermic when it absorbs energy, mostly heat. some examples of endothermic reactions are: in endothermic reactions, more energy is absorbed when the bonds in the reactants. Endothermic Reaction Explanation.
From www.slideserve.com
PPT Endothermic and Exothermic Reactions PowerPoint Presentation Endothermic Reaction Explanation for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. The reaction between ethanoic acid and sodium carbonate. in endothermic reactions, more energy is absorbed when the bonds in the reactants are broken than is released when new bonds are. a chemical reaction is said to be endothermic. Endothermic Reaction Explanation.
From www.slideserve.com
PPT Exothermic and endothermic reactions PowerPoint Presentation Endothermic Reaction Explanation endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. The reaction between ethanoic acid and sodium carbonate. some examples of endothermic reactions are: in endothermic reactions, more energy is absorbed when the bonds in the reactants are broken than is released when new bonds are. The heat is. Endothermic Reaction Explanation.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction Explanation for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from. a chemical reaction is said to be endothermic when it absorbs energy, mostly heat. These reactions lower the. Endothermic Reaction Explanation.
From learningschoolsky17276p.z4.web.core.windows.net
Endothermic And Exothermic Reaction Worksheet Endothermic Reaction Explanation revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from. exothermic and endothermic reactions. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. in endothermic reactions, more energy is absorbed when the bonds in the reactants are. Endothermic Reaction Explanation.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction Explanation exothermic and endothermic reactions. In endothermic and exothermic reactions, energy can be thought of as either. in endothermic reactions, more energy is absorbed when the bonds in the reactants are broken than is released when new bonds are. The heat is absorbed from the surroundings allowing the reactants to transform into. These reactions lower the temperature of their. Endothermic Reaction Explanation.
From www.slideserve.com
PPT Exothermic and endothermic reactions PowerPoint Presentation Endothermic Reaction Explanation some examples of endothermic reactions are: The heat is absorbed from the surroundings allowing the reactants to transform into. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. endothermic reactions are chemical reactions. Endothermic Reaction Explanation.
From wiredatatisse56wr.z22.web.core.windows.net
Endothermic And Exothermic Reaction Diagram Endothermic Reaction Explanation for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. The heat is absorbed from the surroundings allowing the reactants to transform into. revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from. endothermic reactions are chemical reactions. Endothermic Reaction Explanation.
From revisechemistry.uk
Exothermic and Endothermic Reactions AQA C5 revisechemistry.uk Endothermic Reaction Explanation exothermic and endothermic reactions. a chemical reaction is said to be endothermic when it absorbs energy, mostly heat. In endothermic and exothermic reactions, energy can be thought of as either. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. These reactions lower the temperature of their surrounding. Endothermic Reaction Explanation.
From galvinconanstuart.blogspot.com
Energy Diagram Endothermic And Exothermic Reaction General Wiring Diagram Endothermic Reaction Explanation In endothermic and exothermic reactions, energy can be thought of as either. some examples of endothermic reactions are: a chemical reaction is said to be endothermic when it absorbs energy, mostly heat. exothermic and endothermic reactions. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. The. Endothermic Reaction Explanation.
From pediaa.com
Difference Between Endothermic and Exothermic Reactions Definition Endothermic Reaction Explanation These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. some examples of endothermic reactions are: The heat is absorbed from the surroundings allowing the reactants to transform into. The reaction between ethanoic acid and sodium carbonate. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form. Endothermic Reaction Explanation.
From www.slideserve.com
PPT Exothermic and endothermic reactions PowerPoint Presentation Endothermic Reaction Explanation revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. exothermic and endothermic reactions. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their. Endothermic Reaction Explanation.
From lessonfullexcerption.z19.web.core.windows.net
Endothermic And Exothermic Explained Endothermic Reaction Explanation These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. The reaction between ethanoic acid and sodium carbonate. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted. Endothermic Reaction Explanation.
From signalticket9.pythonanywhere.com
Simple Endothermic Reactions With Equations Reaction Balanced Equation Endothermic Reaction Explanation In endothermic and exothermic reactions, energy can be thought of as either. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. in endothermic reactions, more energy is absorbed when the bonds in the reactants are broken than is released when new bonds are. revise and understand what endothermic and exothermic reactions are and. Endothermic Reaction Explanation.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction Explanation endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. some examples of endothermic reactions are: revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from. exothermic and endothermic reactions. In endothermic and exothermic reactions, energy can be. Endothermic Reaction Explanation.
From circuitengineragers88.z22.web.core.windows.net
Energy Diagram For An Endothermic Reaction Endothermic Reaction Explanation a chemical reaction is said to be endothermic when it absorbs energy, mostly heat. In endothermic and exothermic reactions, energy can be thought of as either. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. for an endothermic chemical reaction to proceed, the reactants must absorb energy from. Endothermic Reaction Explanation.
From mareeromana.blogspot.com
12+ Endothermic Enthalpy Diagram MareeRomana Endothermic Reaction Explanation a chemical reaction is said to be endothermic when it absorbs energy, mostly heat. The reaction between ethanoic acid and sodium carbonate. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. in endothermic reactions, more energy is absorbed when the bonds in the reactants are broken than is. Endothermic Reaction Explanation.
From testbook.com
Endothermic Reaction Learn Definition, Reagents, Formula here Endothermic Reaction Explanation revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to.. Endothermic Reaction Explanation.
From www.tes.com
Endothermic and Exothermic Temperature Changes Edexcel 91 Teaching Endothermic Reaction Explanation a chemical reaction is said to be endothermic when it absorbs energy, mostly heat. The reaction between ethanoic acid and sodium carbonate. exothermic and endothermic reactions. in endothermic reactions, more energy is absorbed when the bonds in the reactants are broken than is released when new bonds are. endothermic reactions are chemical reactions in which the. Endothermic Reaction Explanation.
From education-portal.com
Endothermic Reaction Definition & Example Video & Lesson Transcript Endothermic Reaction Explanation In endothermic and exothermic reactions, energy can be thought of as either. The reaction between ethanoic acid and sodium carbonate. some examples of endothermic reactions are: for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. exothermic and endothermic reactions. a chemical reaction is said to be. Endothermic Reaction Explanation.
From www.youtube.com
Endothermic Reactions YouTube Endothermic Reaction Explanation revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from. In endothermic and exothermic reactions, energy can be thought of as either. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. a chemical reaction is said to. Endothermic Reaction Explanation.
From www.vedantu.com
Explain Endothermic Reaction with examples? Endothermic Reaction Explanation some examples of endothermic reactions are: exothermic and endothermic reactions. The reaction between ethanoic acid and sodium carbonate. a chemical reaction is said to be endothermic when it absorbs energy, mostly heat. in endothermic reactions, more energy is absorbed when the bonds in the reactants are broken than is released when new bonds are. These reactions. Endothermic Reaction Explanation.
From sciencenotes.org
Endothermic Reactions Definition and Examples Endothermic Reaction Explanation a chemical reaction is said to be endothermic when it absorbs energy, mostly heat. some examples of endothermic reactions are: for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. The reaction between ethanoic acid and sodium carbonate. These reactions lower the temperature of their surrounding area, thereby. Endothermic Reaction Explanation.
From diagramlistindraught.z13.web.core.windows.net
Enthalpy Diagram For Endothermic Reaction Endothermic Reaction Explanation The reaction between ethanoic acid and sodium carbonate. In endothermic and exothermic reactions, energy can be thought of as either. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to. some examples of endothermic reactions are: in endothermic reactions, more energy is absorbed when the bonds in the. Endothermic Reaction Explanation.
From cexqwbtk.blob.core.windows.net
Endothermic Reaction Synthesis at John Dixon blog Endothermic Reaction Explanation These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. The reaction between ethanoic acid and sodium carbonate. revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from. In endothermic and exothermic reactions, energy can be thought of as either. for an endothermic chemical. Endothermic Reaction Explanation.
From www.slideserve.com
PPT Endothermic Vs. Exothermic Reaction Graphs PowerPoint Endothermic Reaction Explanation The heat is absorbed from the surroundings allowing the reactants to transform into. exothermic and endothermic reactions. In endothermic and exothermic reactions, energy can be thought of as either. a chemical reaction is said to be endothermic when it absorbs energy, mostly heat. some examples of endothermic reactions are: revise and understand what endothermic and exothermic. Endothermic Reaction Explanation.
From www.aakash.ac.in
What are difference between exothermic and endothermic reactions Endothermic Reaction Explanation The reaction between ethanoic acid and sodium carbonate. endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. The heat is absorbed from the surroundings allowing the reactants to transform into. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to.. Endothermic Reaction Explanation.
From www.worksheetsplanet.com
What is an Endothermic Reaction Definition & Example Endothermic Reaction Explanation revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from. a chemical reaction is said to be endothermic when it absorbs energy, mostly heat. in endothermic reactions, more energy is absorbed when the bonds in the reactants are broken than is released when new bonds are. . Endothermic Reaction Explanation.
From classnotes123.com
What does one mean by exothermic and endothermic reactions? Give Endothermic Reaction Explanation In endothermic and exothermic reactions, energy can be thought of as either. The reaction between ethanoic acid and sodium carbonate. revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from. for an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted. Endothermic Reaction Explanation.