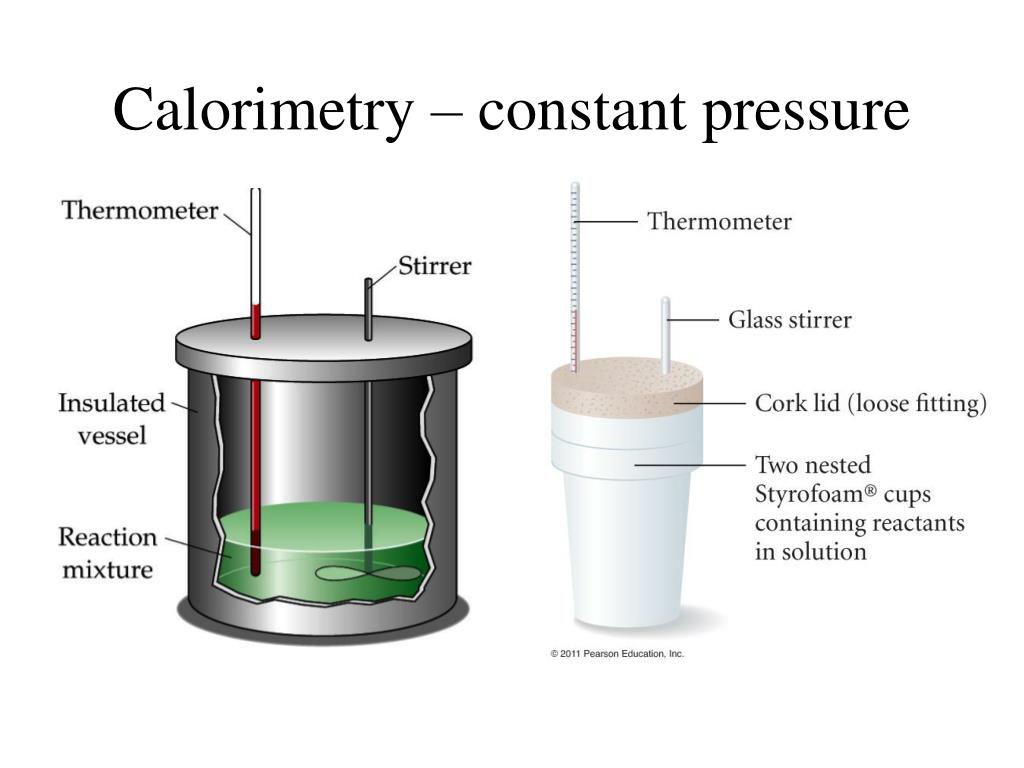
What Is A Perfect Calorimeter . Apply the first law of thermodynamics to calorimetry. For example, when an exothermic. In this article, we will. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction occurs in solution in a. With a calorimeter, you can measure reaction enthalpies or heat capacities using the final temperature (tf) of the contents. Calorimeters have been designed in great variety. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Compare heat flow from hot to cold objects in an ideal. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction.
from www.slideserve.com
For example, when an exothermic reaction occurs in solution in a. Compare heat flow from hot to cold objects in an ideal. For example, when an exothermic. In this article, we will. Apply the first law of thermodynamics to calorimetry. With a calorimeter, you can measure reaction enthalpies or heat capacities using the final temperature (tf) of the contents. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeters have been designed in great variety.
PPT Thermochemistry PowerPoint Presentation, free download ID2692866
What Is A Perfect Calorimeter With a calorimeter, you can measure reaction enthalpies or heat capacities using the final temperature (tf) of the contents. Apply the first law of thermodynamics to calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Compare heat flow from hot to cold objects in an ideal. For example, when an exothermic reaction occurs in solution in a. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. In this article, we will. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeters have been designed in great variety. With a calorimeter, you can measure reaction enthalpies or heat capacities using the final temperature (tf) of the contents.
From www.youtube.com
Thermochemistry ConstantVolume Calorimeter (Bomb Calorimeter). YouTube What Is A Perfect Calorimeter Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Calorimeters have been designed in great variety. For example, when an exothermic. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. For example, when an exothermic. What Is A Perfect Calorimeter.
From www.artisantg.com
IKA C 2000 (Basic Version 1 Calorimeter) ArtisanTG™ What Is A Perfect Calorimeter A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. In this article, we will. Calorimeters have been designed in great variety. For example, when an exothermic reaction occurs in solution in a. Apply the first law of thermodynamics to calorimetry. With a calorimeter, you can measure reaction enthalpies or heat. What Is A Perfect Calorimeter.
From www.tessshebaylo.com
Equation For Determining Calorimetry Tessshebaylo What Is A Perfect Calorimeter Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. In this article, we will. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. For example, when an exothermic reaction occurs in solution in a. For. What Is A Perfect Calorimeter.
From ecampusontario.pressbooks.pub
3.5 Calorimétrie La Chimie Générale pour les GeeGees What Is A Perfect Calorimeter Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. With a calorimeter, you can measure reaction enthalpies or heat capacities using the final temperature (tf) of. What Is A Perfect Calorimeter.
From www.thoughtco.com
Calorimeter Definition in Chemistry What Is A Perfect Calorimeter Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. For example, when an exothermic. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. Compare heat flow from hot to cold objects in an ideal. With. What Is A Perfect Calorimeter.
From www.animalia-life.club
Calorimeter Diagram What Is A Perfect Calorimeter For example, when an exothermic. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeters have been designed in great variety. In this article, we will. A calorimeter. What Is A Perfect Calorimeter.
From mechasource.blogspot.com
An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas What Is A Perfect Calorimeter In this article, we will. For example, when an exothermic. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Apply the first law of thermodynamics to calorimetry. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical. What Is A Perfect Calorimeter.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 What Is A Perfect Calorimeter With a calorimeter, you can measure reaction enthalpies or heat capacities using the final temperature (tf) of the contents. Compare heat flow from hot to cold objects in an ideal. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. For example, when an exothermic reaction. What Is A Perfect Calorimeter.
From wisc.pb.unizin.org
Calorimetry continued Types of Calorimeters and Analyzing Heat Flow What Is A Perfect Calorimeter Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeters. What Is A Perfect Calorimeter.
From punchlistzero.com
Is Heat a State Function? Punchlist Zero What Is A Perfect Calorimeter Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. Apply the first law of thermodynamics to calorimetry. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. In this article, we will. A calorimeter is a. What Is A Perfect Calorimeter.
From thermonine92.blogspot.com
Thermochemistry Calorimeter What Is A Perfect Calorimeter Calorimeters have been designed in great variety. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. For example, when an exothermic. A calorimeter is a device. What Is A Perfect Calorimeter.
From www.chegg.com
Solved In the laboratory a "coffee cup" calorimeter, or What Is A Perfect Calorimeter For example, when an exothermic reaction occurs in solution in a. With a calorimeter, you can measure reaction enthalpies or heat capacities using the final temperature (tf) of the contents. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. Compare heat flow from hot to cold objects in. What Is A Perfect Calorimeter.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID2692866 What Is A Perfect Calorimeter A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. In this article, we will. For example, when an exothermic reaction occurs in solution in a. For example, when an exothermic. A. What Is A Perfect Calorimeter.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube What Is A Perfect Calorimeter Apply the first law of thermodynamics to calorimetry. For example, when an exothermic. For example, when an exothermic reaction occurs in solution in a. Calorimeters have been designed in great variety. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. In this article, we will. A calorimeter. What Is A Perfect Calorimeter.
From www.chegg.com
Solved Calorimetry Calorimetry is the act of measuring heat What Is A Perfect Calorimeter A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Apply the first law of thermodynamics to calorimetry. In this article, we will. A calorimeter is a device used. What Is A Perfect Calorimeter.
From studylib.net
Calorimetry What Is A Perfect Calorimeter Calorimeters have been designed in great variety. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Apply the first law of thermodynamics to calorimetry. In this article, we will. With a calorimeter, you can measure reaction enthalpies or heat capacities using the final temperature (tf) of the. What Is A Perfect Calorimeter.
From slideplayer.com
Calorimeter Experiment to Determine the Specific Heat of Aluminum ppt What Is A Perfect Calorimeter Apply the first law of thermodynamics to calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction occurs in solution in a. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow. What Is A Perfect Calorimeter.
From www.shaalaa.com
Explain the construction of a calorimeter. Draw the necessary figure What Is A Perfect Calorimeter Compare heat flow from hot to cold objects in an ideal. In this article, we will. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Apply the first law of thermodynamics. What Is A Perfect Calorimeter.
From users.highland.edu
Calorimetry What Is A Perfect Calorimeter A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. In this article, we will. With a calorimeter, you can measure reaction enthalpies or heat capacities using the final temperature (tf) of the contents. For example, when an exothermic reaction occurs in solution in a. Calorimeters have been designed in great. What Is A Perfect Calorimeter.
From www.animalia-life.club
Calorimeter Diagram What Is A Perfect Calorimeter A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal. With a calorimeter, you can measure reaction enthalpies or heat capacities using the final temperature (tf) of the contents. Calorimeter, device for. What Is A Perfect Calorimeter.
From courses.lumenlearning.com
Calorimetry Chemistry Atoms First What Is A Perfect Calorimeter A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction occurs in solution in a. In this article, we will. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used. What Is A Perfect Calorimeter.
From labsuppliesusa.com
Calorimeter Electric KLM Bio Scientific What Is A Perfect Calorimeter For example, when an exothermic reaction occurs in solution in a. With a calorimeter, you can measure reaction enthalpies or heat capacities using the final temperature (tf) of the contents. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Apply the first law of thermodynamics to calorimetry. For example, when. What Is A Perfect Calorimeter.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry What Is A Perfect Calorimeter For example, when an exothermic reaction occurs in solution in a. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. In this article, we will. Apply the first law of thermodynamics to calorimetry. For example, when an exothermic. A calorimeter is a device used to measure the. What Is A Perfect Calorimeter.
From www.youtube.com
050 Calorimetry YouTube What Is A Perfect Calorimeter With a calorimeter, you can measure reaction enthalpies or heat capacities using the final temperature (tf) of the contents. For example, when an exothermic reaction occurs in solution in a. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of. What Is A Perfect Calorimeter.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3850751 What Is A Perfect Calorimeter A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. With a calorimeter, you can measure reaction enthalpies or heat capacities using the final temperature (tf) of the contents. For example, when an exothermic reaction occurs in solution in a. A calorimeter is a device used to measure the amount of. What Is A Perfect Calorimeter.
From saylordotorg.github.io
Calorimetry What Is A Perfect Calorimeter A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. For example, when an exothermic. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and. What Is A Perfect Calorimeter.
From www.freeastroscience.com
Exploring Calorimetry A Journey into Heat Transfer Measurement What Is A Perfect Calorimeter Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. For example, when an exothermic. Calorimeters have been designed in great variety. Compare heat flow from hot to cold objects in an ideal. Apply the first law of thermodynamics to calorimetry. A calorimeter is a device used to measure. What Is A Perfect Calorimeter.
From studylib.net
Calorimetry What Is A Perfect Calorimeter For example, when an exothermic reaction occurs in solution in a. Compare heat flow from hot to cold objects in an ideal. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeters have been designed in great variety. In this article, we will. A calorimeter is a device used to. What Is A Perfect Calorimeter.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types What Is A Perfect Calorimeter Compare heat flow from hot to cold objects in an ideal. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. With a calorimeter, you can measure reaction enthalpies or. What Is A Perfect Calorimeter.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry What Is A Perfect Calorimeter Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. Calorimeters have been designed in great variety. In this article, we will. For example, when an exothermic reaction occurs in solution in a. A calorimeter is a device used to measure the amount of heat involved in a chemical. What Is A Perfect Calorimeter.
From www.pinterest.com
Calorimetry Bomb Calorimeter Experiment Science fair, Homeschool and What Is A Perfect Calorimeter Compare heat flow from hot to cold objects in an ideal. For example, when an exothermic reaction occurs in solution in a. With a calorimeter, you can measure reaction enthalpies or heat capacities using the final temperature (tf) of the contents. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a. What Is A Perfect Calorimeter.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation What Is A Perfect Calorimeter For example, when an exothermic reaction occurs in solution in a. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeters have been designed in great variety. With a calorimeter, you can measure reaction enthalpies or heat capacities using the final temperature (tf) of the contents. In this article, we. What Is A Perfect Calorimeter.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts What Is A Perfect Calorimeter A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeters is an important chemistry lab instrument devices that measure the amount of heat absorbed or released during a chemical reaction. Apply the first law of thermodynamics to calorimetry. Calorimeters have been designed in great variety. Calorimeter, device for measuring the. What Is A Perfect Calorimeter.
From www.youtube.com
Principle of Calorimetry YouTube What Is A Perfect Calorimeter In this article, we will. Calorimeters have been designed in great variety. For example, when an exothermic. Compare heat flow from hot to cold objects in an ideal. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical. What Is A Perfect Calorimeter.
From www.youtube.com
Thermochemistry Enthalpy and Coffee Cup Calorimeter. YouTube What Is A Perfect Calorimeter A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. For example, when an exothermic reaction occurs in solution in a. In this article, we will. Compare heat flow from hot to cold objects in an ideal. With a calorimeter, you can measure reaction enthalpies or. What Is A Perfect Calorimeter.