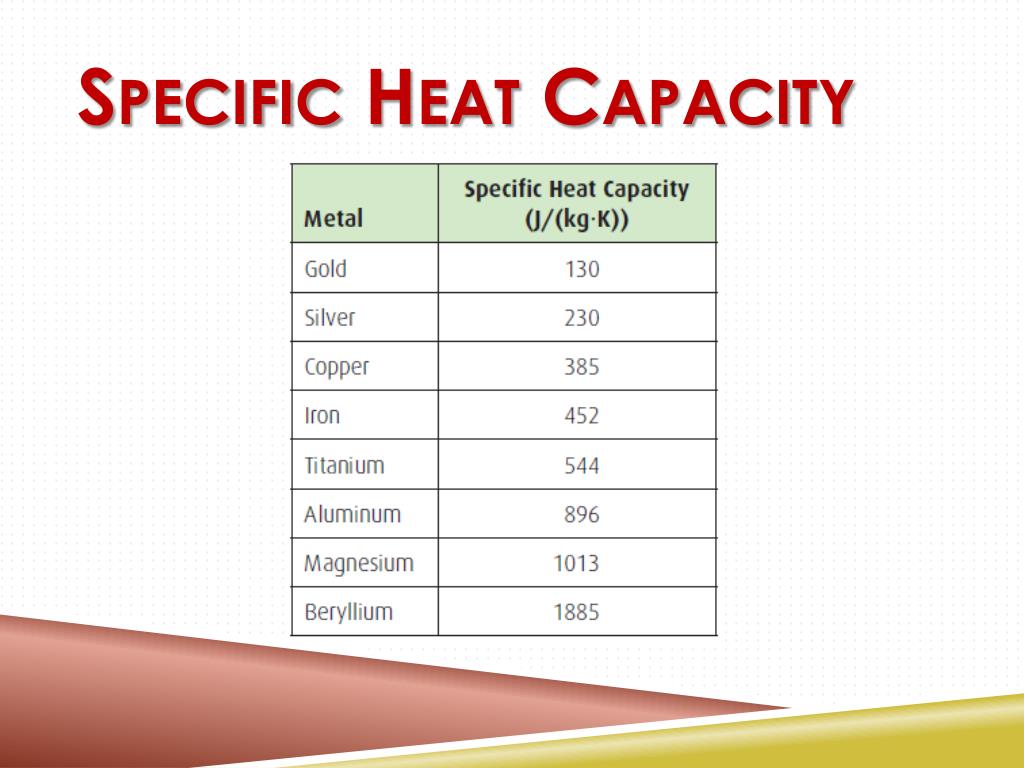
Aluminum Specific Heat . It plays a crucial role in understanding how different materials respond to heating and cooling and describes their ability to store and release thermal energy. Calculate the specific heat of a sample using the formula c = q / (m δ t). Find the specific heat of aluminum and other metals in imperial and si units. Notes on the properties of aluminum:. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Learn how to calculate the heat required to heat a. Click any property name to see plots of that property for all the elements. The web page provides a table. Learn about specific heat, or specific heat capacity, a property related to internal energy in thermodynamics. Find the density, specific heat and thermal conductivity of aluminum at different temperatures. Find typical values of specific heat for various substances, including aluminum (890 j / kg · k).
from ar.inspiredpencil.com
Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Find the specific heat of aluminum and other metals in imperial and si units. Notes on the properties of aluminum:. Learn how to calculate the heat required to heat a. Calculate the specific heat of a sample using the formula c = q / (m δ t). The web page provides a table. Click any property name to see plots of that property for all the elements. Find the density, specific heat and thermal conductivity of aluminum at different temperatures. It plays a crucial role in understanding how different materials respond to heating and cooling and describes their ability to store and release thermal energy. Learn about specific heat, or specific heat capacity, a property related to internal energy in thermodynamics.
Specific Heat Chart Aluminum
Aluminum Specific Heat The web page provides a table. Learn how to calculate the heat required to heat a. Find typical values of specific heat for various substances, including aluminum (890 j / kg · k). Find the density, specific heat and thermal conductivity of aluminum at different temperatures. Calculate the specific heat of a sample using the formula c = q / (m δ t). Learn about specific heat, or specific heat capacity, a property related to internal energy in thermodynamics. It plays a crucial role in understanding how different materials respond to heating and cooling and describes their ability to store and release thermal energy. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Find the specific heat of aluminum and other metals in imperial and si units. The web page provides a table. Notes on the properties of aluminum:. Click any property name to see plots of that property for all the elements.
From
Aluminum Specific Heat It plays a crucial role in understanding how different materials respond to heating and cooling and describes their ability to store and release thermal energy. Learn how to calculate the heat required to heat a. Calculate the specific heat of a sample using the formula c = q / (m δ t). Click any property name to see plots of. Aluminum Specific Heat.
From mavink.com
Aluminum Specific Heat Aluminum Specific Heat It plays a crucial role in understanding how different materials respond to heating and cooling and describes their ability to store and release thermal energy. The web page provides a table. Notes on the properties of aluminum:. Learn about specific heat, or specific heat capacity, a property related to internal energy in thermodynamics. Calculate the specific heat of a sample. Aluminum Specific Heat.
From
Aluminum Specific Heat Calculate the specific heat of a sample using the formula c = q / (m δ t). Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. The web page provides a table. Find the density, specific heat and thermal conductivity of aluminum at different. Aluminum Specific Heat.
From
Aluminum Specific Heat Learn how to calculate the heat required to heat a. It plays a crucial role in understanding how different materials respond to heating and cooling and describes their ability to store and release thermal energy. Click any property name to see plots of that property for all the elements. Find typical values of specific heat for various substances, including aluminum. Aluminum Specific Heat.
From www.tec-science.com
Specific heat capacity of selected substances tecscience Aluminum Specific Heat Click any property name to see plots of that property for all the elements. Notes on the properties of aluminum:. Learn how to calculate the heat required to heat a. The web page provides a table. It plays a crucial role in understanding how different materials respond to heating and cooling and describes their ability to store and release thermal. Aluminum Specific Heat.
From www.chegg.com
Solved A 2.15 kg block of aluminum (specific heat 897 Aluminum Specific Heat Find the density, specific heat and thermal conductivity of aluminum at different temperatures. It plays a crucial role in understanding how different materials respond to heating and cooling and describes their ability to store and release thermal energy. Notes on the properties of aluminum:. Find the specific heat of aluminum and other metals in imperial and si units. Find typical. Aluminum Specific Heat.
From
Aluminum Specific Heat Learn about specific heat, or specific heat capacity, a property related to internal energy in thermodynamics. Learn how to calculate the heat required to heat a. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Click any property name to see plots of that. Aluminum Specific Heat.
From
Aluminum Specific Heat Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Find the specific heat of aluminum and other metals in imperial and si units. Find the density, specific heat and thermal conductivity of aluminum at different temperatures. Notes on the properties of aluminum:. It plays. Aluminum Specific Heat.
From
Aluminum Specific Heat Find the specific heat of aluminum and other metals in imperial and si units. It plays a crucial role in understanding how different materials respond to heating and cooling and describes their ability to store and release thermal energy. The web page provides a table. Notes on the properties of aluminum:. Learn how to calculate the heat required to heat. Aluminum Specific Heat.
From
Aluminum Specific Heat Notes on the properties of aluminum:. Learn how to calculate the heat required to heat a. Find typical values of specific heat for various substances, including aluminum (890 j / kg · k). The web page provides a table. Calculate the specific heat of a sample using the formula c = q / (m δ t). Learn about specific heat,. Aluminum Specific Heat.
From aluminumgenjin.blogspot.com
Aluminum Aluminum Specific Heat Aluminum Specific Heat Learn about specific heat, or specific heat capacity, a property related to internal energy in thermodynamics. Find typical values of specific heat for various substances, including aluminum (890 j / kg · k). Learn how to calculate the heat required to heat a. The web page provides a table. Notes on the properties of aluminum:. Find the density, specific heat. Aluminum Specific Heat.
From
Aluminum Specific Heat Learn how to calculate the heat required to heat a. Click any property name to see plots of that property for all the elements. It plays a crucial role in understanding how different materials respond to heating and cooling and describes their ability to store and release thermal energy. Find the specific heat of aluminum and other metals in imperial. Aluminum Specific Heat.
From
Aluminum Specific Heat Notes on the properties of aluminum:. Click any property name to see plots of that property for all the elements. Learn about specific heat, or specific heat capacity, a property related to internal energy in thermodynamics. Calculate the specific heat of a sample using the formula c = q / (m δ t). Find the specific heat of aluminum and. Aluminum Specific Heat.
From
Aluminum Specific Heat Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Click any property name to see plots of that property for all the elements. Find the density, specific heat and thermal conductivity of aluminum at different temperatures. The web page provides a table. Learn about. Aluminum Specific Heat.
From www.alamy.com
Specific Heat Capacity of Aluminium Experiment Set up with no Aluminum Specific Heat Click any property name to see plots of that property for all the elements. Find typical values of specific heat for various substances, including aluminum (890 j / kg · k). Notes on the properties of aluminum:. Learn about specific heat, or specific heat capacity, a property related to internal energy in thermodynamics. The web page provides a table. It. Aluminum Specific Heat.
From
Aluminum Specific Heat Learn about specific heat, or specific heat capacity, a property related to internal energy in thermodynamics. It plays a crucial role in understanding how different materials respond to heating and cooling and describes their ability to store and release thermal energy. Learn how to calculate the heat required to heat a. Notes on the properties of aluminum:. Find typical values. Aluminum Specific Heat.
From
Aluminum Specific Heat Learn how to calculate the heat required to heat a. The web page provides a table. Notes on the properties of aluminum:. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Find the density, specific heat and thermal conductivity of aluminum at different temperatures.. Aluminum Specific Heat.
From www.studypool.com
SOLUTION Specific heat capacity definition, importance, and formula Aluminum Specific Heat Find typical values of specific heat for various substances, including aluminum (890 j / kg · k). Find the density, specific heat and thermal conductivity of aluminum at different temperatures. Learn how to calculate the heat required to heat a. Calculate the specific heat of a sample using the formula c = q / (m δ t). It plays a. Aluminum Specific Heat.
From learningdbpfeifer.z21.web.core.windows.net
Electrical Conductivity Of Metals Table Aluminum Specific Heat Notes on the properties of aluminum:. Calculate the specific heat of a sample using the formula c = q / (m δ t). Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. The web page provides a table. Find the specific heat of aluminum. Aluminum Specific Heat.
From
Aluminum Specific Heat Find the specific heat of aluminum and other metals in imperial and si units. Learn about specific heat, or specific heat capacity, a property related to internal energy in thermodynamics. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Find the density, specific heat. Aluminum Specific Heat.
From
Aluminum Specific Heat Notes on the properties of aluminum:. Click any property name to see plots of that property for all the elements. Learn about specific heat, or specific heat capacity, a property related to internal energy in thermodynamics. The web page provides a table. Learn how to calculate the heat required to heat a. Find the density, specific heat and thermal conductivity. Aluminum Specific Heat.
From
Aluminum Specific Heat Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Learn about specific heat, or specific heat capacity, a property related to internal energy in thermodynamics. Calculate the specific heat of a sample using the formula c = q / (m δ t). Click any. Aluminum Specific Heat.
From infogram.com
Aluminum Heating Curve Infogram Aluminum Specific Heat Find the specific heat of aluminum and other metals in imperial and si units. The web page provides a table. It plays a crucial role in understanding how different materials respond to heating and cooling and describes their ability to store and release thermal energy. Notes on the properties of aluminum:. Learn how to calculate the heat required to heat. Aluminum Specific Heat.
From
Aluminum Specific Heat Click any property name to see plots of that property for all the elements. Find the density, specific heat and thermal conductivity of aluminum at different temperatures. Find typical values of specific heat for various substances, including aluminum (890 j / kg · k). Learn about specific heat, or specific heat capacity, a property related to internal energy in thermodynamics.. Aluminum Specific Heat.
From www.vrogue.co
Specific Heat Capacity vrogue.co Aluminum Specific Heat Learn how to calculate the heat required to heat a. Find the density, specific heat and thermal conductivity of aluminum at different temperatures. Find the specific heat of aluminum and other metals in imperial and si units. It plays a crucial role in understanding how different materials respond to heating and cooling and describes their ability to store and release. Aluminum Specific Heat.
From
Aluminum Specific Heat Find the density, specific heat and thermal conductivity of aluminum at different temperatures. Find typical values of specific heat for various substances, including aluminum (890 j / kg · k). The web page provides a table. Find the specific heat of aluminum and other metals in imperial and si units. Click any property name to see plots of that property. Aluminum Specific Heat.
From www.ioffe.ru
NSM Archive Aluminium Nitride (AlN) Thermal properties Aluminum Specific Heat Find the specific heat of aluminum and other metals in imperial and si units. Learn about specific heat, or specific heat capacity, a property related to internal energy in thermodynamics. Notes on the properties of aluminum:. Click any property name to see plots of that property for all the elements. Learn how to calculate the heat required to heat a.. Aluminum Specific Heat.
From
Aluminum Specific Heat Find the specific heat of aluminum and other metals in imperial and si units. Learn about specific heat, or specific heat capacity, a property related to internal energy in thermodynamics. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. The web page provides a. Aluminum Specific Heat.
From www.oreilly.com
Appendix G. Heat Capacity Equations Basic Principles and Calculations Aluminum Specific Heat The web page provides a table. Calculate the specific heat of a sample using the formula c = q / (m δ t). Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Learn about specific heat, or specific heat capacity, a property related to. Aluminum Specific Heat.
From www.mdpi.com
Thermal Conductivity of Aluminum Alloys—A Review Aluminum Specific Heat Find the density, specific heat and thermal conductivity of aluminum at different temperatures. It plays a crucial role in understanding how different materials respond to heating and cooling and describes their ability to store and release thermal energy. Learn about specific heat, or specific heat capacity, a property related to internal energy in thermodynamics. The web page provides a table.. Aluminum Specific Heat.
From www.numerade.com
SOLVED Table 4.2 SPECIFIC HEATS OF COMMON SUBSTANCES Material Aluminum Specific Heat Find the density, specific heat and thermal conductivity of aluminum at different temperatures. Notes on the properties of aluminum:. Click any property name to see plots of that property for all the elements. The web page provides a table. Calculate the specific heat of a sample using the formula c = q / (m δ t). Learn how to calculate. Aluminum Specific Heat.
From
Aluminum Specific Heat Calculate the specific heat of a sample using the formula c = q / (m δ t). Notes on the properties of aluminum:. Find typical values of specific heat for various substances, including aluminum (890 j / kg · k). Click any property name to see plots of that property for all the elements. It plays a crucial role in. Aluminum Specific Heat.
From www.numerade.com
SOLVED 'A 89.2 g piece of aluminum (which has molar heat capacity of Aluminum Specific Heat The web page provides a table. Calculate the specific heat of a sample using the formula c = q / (m δ t). Notes on the properties of aluminum:. Click any property name to see plots of that property for all the elements. Find the density, specific heat and thermal conductivity of aluminum at different temperatures. Learn about specific heat,. Aluminum Specific Heat.
From
Aluminum Specific Heat Learn how to calculate the heat required to heat a. Calculate the specific heat of a sample using the formula c = q / (m δ t). Find typical values of specific heat for various substances, including aluminum (890 j / kg · k). Find the specific heat of aluminum and other metals in imperial and si units. Specific heat. Aluminum Specific Heat.
From
Aluminum Specific Heat The web page provides a table. Notes on the properties of aluminum:. Find the specific heat of aluminum and other metals in imperial and si units. Find typical values of specific heat for various substances, including aluminum (890 j / kg · k). Find the density, specific heat and thermal conductivity of aluminum at different temperatures. Learn about specific heat,. Aluminum Specific Heat.