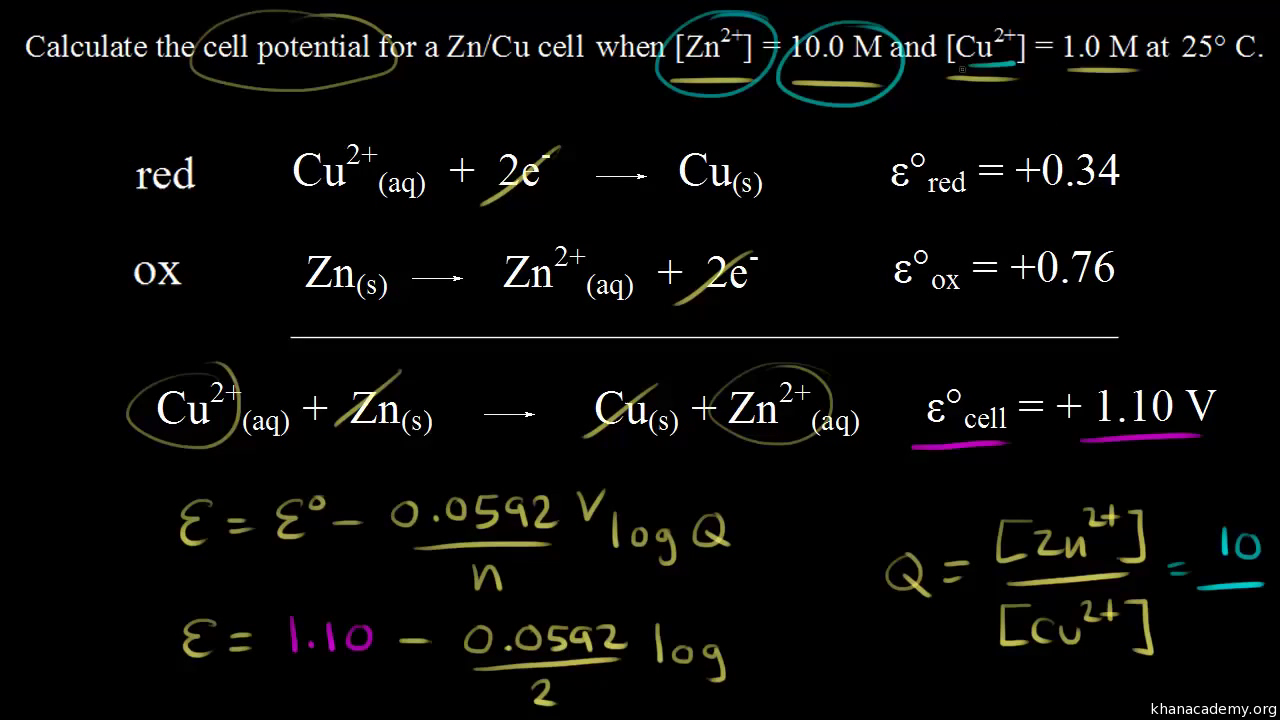Standard Cell Potential Concentration Cell . It has a cell potential of. The standard electrode potential, commonly written as e o cell, of a concentration cell is equal to zero because the electrodes are. Concentration cells consist of anode and cathode compartments that are identical except for the concentrations of the reactant. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. Concentration cells harness ion concentration differences to generate electric potential. This is a cell under nonstandard conditions, and therefore, we are going to use the nernst equation to calculate its potential (e. This chemistry video tutorial provides a basic introduction into concentration cells. Calculating the cell potential with different concentrations. Because δg = 0 at equilibrium, the measured.
from salma-has-blevins.blogspot.com
Calculating the cell potential with different concentrations. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: This is a cell under nonstandard conditions, and therefore, we are going to use the nernst equation to calculate its potential (e. It has a cell potential of. The standard electrode potential, commonly written as e o cell, of a concentration cell is equal to zero because the electrodes are. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. Concentration cells harness ion concentration differences to generate electric potential. Concentration cells consist of anode and cathode compartments that are identical except for the concentrations of the reactant. This chemistry video tutorial provides a basic introduction into concentration cells. Because δg = 0 at equilibrium, the measured.
How to Calculate Cell Potential Under Standard Conditions Salmahas
Standard Cell Potential Concentration Cell Because δg = 0 at equilibrium, the measured. This is a cell under nonstandard conditions, and therefore, we are going to use the nernst equation to calculate its potential (e. It has a cell potential of. Concentration cells consist of anode and cathode compartments that are identical except for the concentrations of the reactant. This chemistry video tutorial provides a basic introduction into concentration cells. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: Concentration cells harness ion concentration differences to generate electric potential. Calculating the cell potential with different concentrations. Because δg = 0 at equilibrium, the measured. The standard electrode potential, commonly written as e o cell, of a concentration cell is equal to zero because the electrodes are.
From www.slideserve.com
PPT 14.2b Standard Cells and Cell Potential PowerPoint Presentation Standard Cell Potential Concentration Cell Because δg = 0 at equilibrium, the measured. Concentration cells harness ion concentration differences to generate electric potential. This is a cell under nonstandard conditions, and therefore, we are going to use the nernst equation to calculate its potential (e. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: 1 atm for. Standard Cell Potential Concentration Cell.
From www.slideserve.com
PPT 14.2b Standard Cells and Cell Potential PowerPoint Presentation Standard Cell Potential Concentration Cell Concentration cells consist of anode and cathode compartments that are identical except for the concentrations of the reactant. This is a cell under nonstandard conditions, and therefore, we are going to use the nernst equation to calculate its potential (e. It has a cell potential of. Calculating the cell potential with different concentrations. Because δg = 0 at equilibrium, the. Standard Cell Potential Concentration Cell.
From www.expii.com
Concentration Cells — Overview & Applications Expii Standard Cell Potential Concentration Cell Concentration cells consist of anode and cathode compartments that are identical except for the concentrations of the reactant. Concentration cells harness ion concentration differences to generate electric potential. Because δg = 0 at equilibrium, the measured. This is a cell under nonstandard conditions, and therefore, we are going to use the nernst equation to calculate its potential (e. 1 atm. Standard Cell Potential Concentration Cell.
From www.youtube.com
Calculating the Cell Potential of Electrochemical Cells. (Adv Chem Ch Standard Cell Potential Concentration Cell 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. Calculating the cell potential with different concentrations. Because δg = 0 at equilibrium, the measured. It has a cell potential of. This is a cell under nonstandard conditions, and therefore, we are going to use the nernst equation to calculate its potential (e. This chemistry video. Standard Cell Potential Concentration Cell.
From www.slideserve.com
PPT Chemistry 232 PowerPoint Presentation, free download ID942926 Standard Cell Potential Concentration Cell The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: This is a cell under nonstandard conditions, and therefore, we are going to use the nernst equation to calculate its potential (e. This chemistry video tutorial provides a basic introduction into concentration cells. Concentration cells harness ion concentration differences to generate electric potential.. Standard Cell Potential Concentration Cell.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Standard Cell Potential Concentration Cell This chemistry video tutorial provides a basic introduction into concentration cells. Concentration cells consist of anode and cathode compartments that are identical except for the concentrations of the reactant. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. Concentration cells harness ion concentration differences to generate electric potential. It has a cell potential of. The. Standard Cell Potential Concentration Cell.
From salma-has-blevins.blogspot.com
How to Calculate Cell Potential Under Standard Conditions Salmahas Standard Cell Potential Concentration Cell Calculating the cell potential with different concentrations. It has a cell potential of. This chemistry video tutorial provides a basic introduction into concentration cells. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: Because δg = 0 at equilibrium, the measured. The standard electrode potential, commonly written as e o cell, of. Standard Cell Potential Concentration Cell.
From www.slideserve.com
PPT Chapter 17 Electrochemistry PowerPoint Presentation, free Standard Cell Potential Concentration Cell Because δg = 0 at equilibrium, the measured. This chemistry video tutorial provides a basic introduction into concentration cells. Calculating the cell potential with different concentrations. Concentration cells harness ion concentration differences to generate electric potential. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: The standard electrode potential, commonly written as. Standard Cell Potential Concentration Cell.
From www.youtube.com
STANDARD CELL POTENTIAL YouTube Standard Cell Potential Concentration Cell Concentration cells harness ion concentration differences to generate electric potential. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: The standard electrode potential, commonly written as e o cell, of a concentration cell is equal to zero because the electrodes are. This chemistry video tutorial provides a basic introduction into concentration cells.. Standard Cell Potential Concentration Cell.
From general.chemistrysteps.com
Cell Potential, Free Energy, and Equilibrium Constant Chemistry Steps Standard Cell Potential Concentration Cell The standard electrode potential, commonly written as e o cell, of a concentration cell is equal to zero because the electrodes are. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. This is a cell under nonstandard conditions, and therefore, we are going to use the nernst equation to calculate its potential (e. Because δg. Standard Cell Potential Concentration Cell.
From www.youtube.com
Standard Cell Potentials YouTube Standard Cell Potential Concentration Cell The standard electrode potential, commonly written as e o cell, of a concentration cell is equal to zero because the electrodes are. Concentration cells consist of anode and cathode compartments that are identical except for the concentrations of the reactant. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. It has a cell potential of.. Standard Cell Potential Concentration Cell.
From www.differencebetween.com
Difference Between Concentration Cell and Chemical Cell Compare the Standard Cell Potential Concentration Cell This is a cell under nonstandard conditions, and therefore, we are going to use the nernst equation to calculate its potential (e. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: Calculating the cell potential with different concentrations.. Standard Cell Potential Concentration Cell.
From general.chemistrysteps.com
How to Calculate Standard Cell Potential Chemistry Steps Standard Cell Potential Concentration Cell This chemistry video tutorial provides a basic introduction into concentration cells. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: Concentration cells consist of anode and cathode compartments that are identical except for the concentrations of the reactant. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes.. Standard Cell Potential Concentration Cell.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID1195570 Standard Cell Potential Concentration Cell This chemistry video tutorial provides a basic introduction into concentration cells. Calculating the cell potential with different concentrations. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. It has a cell potential of. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: Because δg = 0 at. Standard Cell Potential Concentration Cell.
From www.nagwa.com
Question Video Calculating a Standard Cell Potential from Standard Standard Cell Potential Concentration Cell Concentration cells consist of anode and cathode compartments that are identical except for the concentrations of the reactant. Because δg = 0 at equilibrium, the measured. The standard electrode potential, commonly written as e o cell, of a concentration cell is equal to zero because the electrodes are. Concentration cells harness ion concentration differences to generate electric potential. This is. Standard Cell Potential Concentration Cell.
From byjus.com
Variation of Cell Potential in ZnCu Cell Chemistry Practicals Class Standard Cell Potential Concentration Cell Concentration cells consist of anode and cathode compartments that are identical except for the concentrations of the reactant. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: It has a cell potential of. Because δg = 0 at equilibrium, the measured. This is a cell under nonstandard conditions, and therefore, we are. Standard Cell Potential Concentration Cell.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Standard Cell Potential Concentration Cell The standard electrode potential, commonly written as e o cell, of a concentration cell is equal to zero because the electrodes are. It has a cell potential of. Concentration cells harness ion concentration differences to generate electric potential. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. This is a cell under nonstandard conditions, and. Standard Cell Potential Concentration Cell.
From chemwiki.ucdavis.edu
Standard Potentials Chemwiki Standard Cell Potential Concentration Cell The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. This is a cell under nonstandard conditions, and therefore, we are going to use the nernst equation to calculate its potential (e. The standard electrode potential, commonly written as. Standard Cell Potential Concentration Cell.
From www.bartleby.com
Answered The standard cell potential (E° cell)… bartleby Standard Cell Potential Concentration Cell Because δg = 0 at equilibrium, the measured. Concentration cells consist of anode and cathode compartments that are identical except for the concentrations of the reactant. The standard electrode potential, commonly written as e o cell, of a concentration cell is equal to zero because the electrodes are. Concentration cells harness ion concentration differences to generate electric potential. The standard. Standard Cell Potential Concentration Cell.
From www.youtube.com
The Nernst Equation and Concentration Cells Plus Examples YouTube Standard Cell Potential Concentration Cell This is a cell under nonstandard conditions, and therefore, we are going to use the nernst equation to calculate its potential (e. Concentration cells consist of anode and cathode compartments that are identical except for the concentrations of the reactant. This chemistry video tutorial provides a basic introduction into concentration cells. Concentration cells harness ion concentration differences to generate electric. Standard Cell Potential Concentration Cell.
From pdfslide.net
(PPT) Electrochemistry Electrochemical Cells Voltaic Cells Standard Standard Cell Potential Concentration Cell 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. Concentration cells consist of anode and cathode compartments that are identical except for the concentrations of the reactant. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: The standard electrode potential, commonly written as e o cell, of. Standard Cell Potential Concentration Cell.
From courses.lumenlearning.com
17.3 Standard Reduction Potentials General College Chemistry II Standard Cell Potential Concentration Cell Concentration cells consist of anode and cathode compartments that are identical except for the concentrations of the reactant. This chemistry video tutorial provides a basic introduction into concentration cells. It has a cell potential of. Concentration cells harness ion concentration differences to generate electric potential. Because δg = 0 at equilibrium, the measured. The standard electrode potential, commonly written as. Standard Cell Potential Concentration Cell.
From www.youtube.com
Calculating the equilibrium constant from the standard cell potential Standard Cell Potential Concentration Cell 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. Because δg = 0 at equilibrium, the measured. This chemistry video tutorial provides a basic introduction into concentration cells. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: The standard electrode potential, commonly written as e o cell,. Standard Cell Potential Concentration Cell.
From halleldmoses.blogspot.com
Cell Potential Formula HalleldMoses Standard Cell Potential Concentration Cell Concentration cells harness ion concentration differences to generate electric potential. Because δg = 0 at equilibrium, the measured. Concentration cells consist of anode and cathode compartments that are identical except for the concentrations of the reactant. The standard electrode potential, commonly written as e o cell, of a concentration cell is equal to zero because the electrodes are. 1 atm. Standard Cell Potential Concentration Cell.
From inspiritvr.com
Calculating Standard Cell Potentials Study Guide Inspirit Standard Cell Potential Concentration Cell This is a cell under nonstandard conditions, and therefore, we are going to use the nernst equation to calculate its potential (e. Calculating the cell potential with different concentrations. The standard electrode potential, commonly written as e o cell, of a concentration cell is equal to zero because the electrodes are. This chemistry video tutorial provides a basic introduction into. Standard Cell Potential Concentration Cell.
From chem.libretexts.org
20.4 Cell Potential Under Standard Conditions Chemistry LibreTexts Standard Cell Potential Concentration Cell Calculating the cell potential with different concentrations. This is a cell under nonstandard conditions, and therefore, we are going to use the nernst equation to calculate its potential (e. Because δg = 0 at equilibrium, the measured. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. The standard cell potential (e°cell) is measured at 298. Standard Cell Potential Concentration Cell.
From www.youtube.com
Cell Concentration and the Nernst Equation YouTube Standard Cell Potential Concentration Cell Because δg = 0 at equilibrium, the measured. Concentration cells harness ion concentration differences to generate electric potential. This is a cell under nonstandard conditions, and therefore, we are going to use the nernst equation to calculate its potential (e. The standard electrode potential, commonly written as e o cell, of a concentration cell is equal to zero because the. Standard Cell Potential Concentration Cell.
From www.slideserve.com
PPT Electrochemistry szczepascanisius.edu PowerPoint Presentation Standard Cell Potential Concentration Cell 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. Because δg = 0 at equilibrium, the measured. Concentration cells consist of anode and cathode compartments that are identical except for the concentrations of the reactant. This chemistry video tutorial provides a basic introduction into concentration cells. It has a cell potential of. Calculating the cell. Standard Cell Potential Concentration Cell.
From www.slideserve.com
PPT 14.2b Standard Cells and Cell Potential PowerPoint Presentation Standard Cell Potential Concentration Cell This is a cell under nonstandard conditions, and therefore, we are going to use the nernst equation to calculate its potential (e. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: It has a cell potential of. Because. Standard Cell Potential Concentration Cell.
From slideplayer.com
Equilibrium Electrochemistry ppt download Standard Cell Potential Concentration Cell Concentration cells harness ion concentration differences to generate electric potential. Calculating the cell potential with different concentrations. This chemistry video tutorial provides a basic introduction into concentration cells. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. It has a cell potential of. This is a cell under nonstandard conditions, and therefore, we are going. Standard Cell Potential Concentration Cell.
From www.slideserve.com
PPT Nernst Equation PowerPoint Presentation, free download ID6621500 Standard Cell Potential Concentration Cell Because δg = 0 at equilibrium, the measured. The standard electrode potential, commonly written as e o cell, of a concentration cell is equal to zero because the electrodes are. Concentration cells harness ion concentration differences to generate electric potential. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: This is a. Standard Cell Potential Concentration Cell.
From quizdbbarnstorms.z21.web.core.windows.net
What Is Standard Cell Potential Standard Cell Potential Concentration Cell 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. This is a cell under nonstandard conditions, and therefore, we are going to use the nernst equation to calculate its potential (e. Concentration cells harness ion concentration differences to generate electric potential. Concentration cells consist of anode and cathode compartments that are identical except for the. Standard Cell Potential Concentration Cell.
From www.chegg.com
Solved Use the halfreactions below to produce a voltaic Standard Cell Potential Concentration Cell Concentration cells harness ion concentration differences to generate electric potential. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: Calculating the cell potential with different concentrations. Concentration cells consist of anode and cathode compartments that are identical except for the concentrations of the reactant. Because δg = 0 at equilibrium, the measured.. Standard Cell Potential Concentration Cell.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID7041672 Standard Cell Potential Concentration Cell Concentration cells harness ion concentration differences to generate electric potential. Concentration cells consist of anode and cathode compartments that are identical except for the concentrations of the reactant. This is a cell under nonstandard conditions, and therefore, we are going to use the nernst equation to calculate its potential (e. Because δg = 0 at equilibrium, the measured. The standard. Standard Cell Potential Concentration Cell.
From general.chemistrysteps.com
Concentration Cells Chemistry Steps Standard Cell Potential Concentration Cell The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: Because δg = 0 at equilibrium, the measured. Calculating the cell potential with different concentrations. Concentration cells consist of anode and cathode compartments that are identical except for the concentrations of the reactant. The standard electrode potential, commonly written as e o cell,. Standard Cell Potential Concentration Cell.