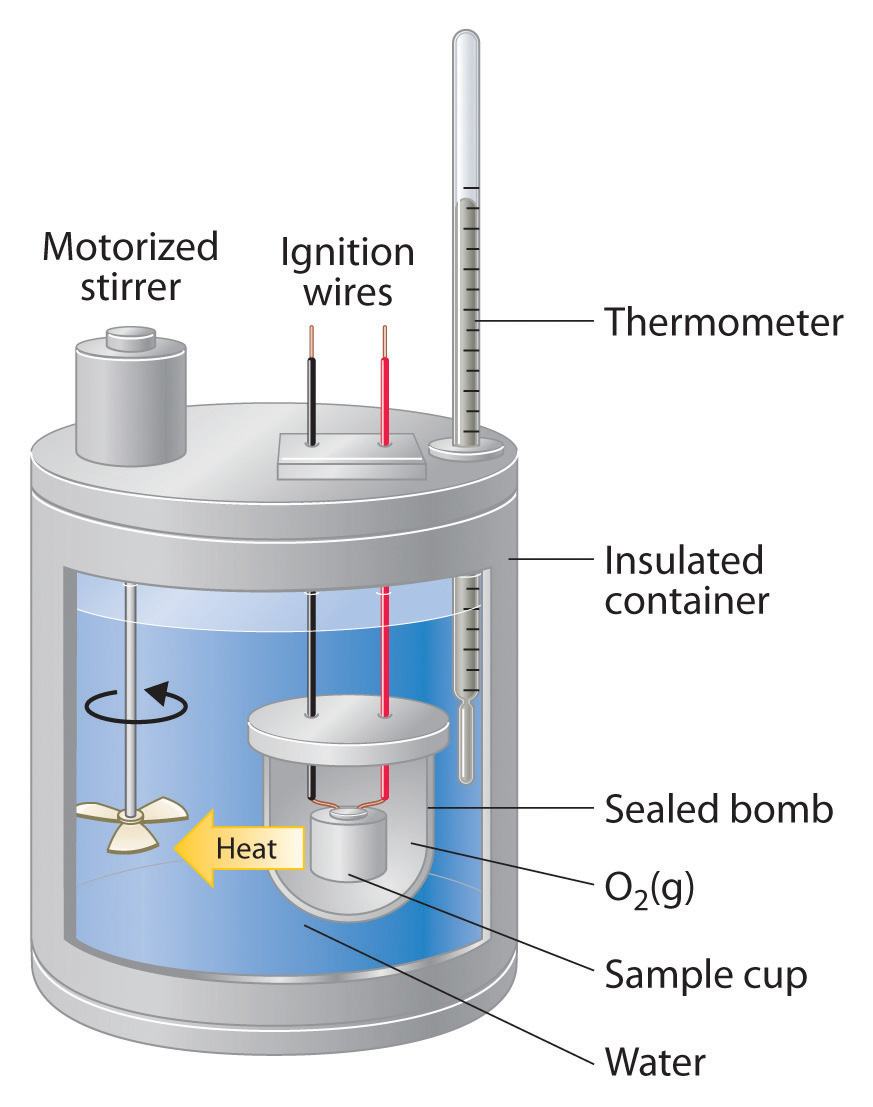
Bomb Calorimeter Chemistry . Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. A tutorial guide on how to calculate the heat of combustion. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. This type of calorimeter consists of a robust steel container (the “bomb”) that contains the reactants and is itself submerged in water (figure 5.17). This lab demonstrates one of the most common. Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. So, for a constant volume, δe = q, and therefore, the bomb calorimeter measures the energy change of a chemical reaction. A bomb calorimeter is an instrument used to determine the heat emitted from a given quantity of biomass sample combustion and to calculate the hhv of that biomass fuel. Compare and contrast coffee cup calorimetry and bomb calorimetry.
from saylordotorg.github.io
So, for a constant volume, δe = q, and therefore, the bomb calorimeter measures the energy change of a chemical reaction. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. Compare and contrast coffee cup calorimetry and bomb calorimetry. A tutorial guide on how to calculate the heat of combustion. This lab demonstrates one of the most common. This type of calorimeter consists of a robust steel container (the “bomb”) that contains the reactants and is itself submerged in water (figure 5.17). A bomb calorimeter is an instrument used to determine the heat emitted from a given quantity of biomass sample combustion and to calculate the hhv of that biomass fuel. Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter.
Calorimetry
Bomb Calorimeter Chemistry Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. So, for a constant volume, δe = q, and therefore, the bomb calorimeter measures the energy change of a chemical reaction. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Compare and contrast coffee cup calorimetry and bomb calorimetry. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. A bomb calorimeter is an instrument used to determine the heat emitted from a given quantity of biomass sample combustion and to calculate the hhv of that biomass fuel. A tutorial guide on how to calculate the heat of combustion. This type of calorimeter consists of a robust steel container (the “bomb”) that contains the reactants and is itself submerged in water (figure 5.17). This lab demonstrates one of the most common.
From www.education.com
Calorimetry Bomb Calorimeter Experiment Bomb Calorimeter Chemistry So, for a constant volume, δe = q, and therefore, the bomb calorimeter measures the energy change of a chemical reaction. Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. A bomb calorimeter is an instrument used to determine the heat emitted from a given quantity of biomass sample combustion and to. Bomb Calorimeter Chemistry.
From www.youtube.com
Bomb Calorimeter Experiment Calorific Value of Solid Fuel by Bomb Calorimeter YouTube Bomb Calorimeter Chemistry This type of calorimeter consists of a robust steel container (the “bomb”) that contains the reactants and is itself submerged in water (figure 5.17). Compare and contrast coffee cup calorimetry and bomb calorimetry. A tutorial guide on how to calculate the heat of combustion. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in. Bomb Calorimeter Chemistry.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID2692866 Bomb Calorimeter Chemistry This type of calorimeter consists of a robust steel container (the “bomb”) that contains the reactants and is itself submerged in water (figure 5.17). So, for a constant volume, δe = q, and therefore, the bomb calorimeter measures the energy change of a chemical reaction. A tutorial guide on how to calculate the heat of combustion. This lab demonstrates one. Bomb Calorimeter Chemistry.
From www.vrogue.co
What Is Calorimetry With Pictures vrogue.co Bomb Calorimeter Chemistry So, for a constant volume, δe = q, and therefore, the bomb calorimeter measures the energy change of a chemical reaction. This type of calorimeter consists of a robust steel container (the “bomb”) that contains the reactants and is itself submerged in water (figure 5.17). Describe a simple calorimeter and explain how it is employed and how its heat capacity. Bomb Calorimeter Chemistry.
From www.slideserve.com
PPT AP Chemistry Unit 7 Thermodynamics PowerPoint Presentation, free download ID5574364 Bomb Calorimeter Chemistry Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. This lab demonstrates one of the most common. So, for a constant volume, δe = q, and therefore, the bomb calorimeter measures the energy change of a chemical reaction. This type of calorimeter consists of a robust steel container (the “bomb”) that. Bomb Calorimeter Chemistry.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii Bomb Calorimeter Chemistry Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. So, for a constant volume, δe = q, and therefore, the bomb calorimeter measures the energy change of a chemical reaction. This lab demonstrates one of the most common. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. Calculate. Bomb Calorimeter Chemistry.
From chem.libretexts.org
11.5 Reaction Calorimetry Chemistry LibreTexts Bomb Calorimeter Chemistry Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. Compare and contrast coffee cup calorimetry and bomb calorimetry. So, for a constant volume, δe = q, and therefore, the bomb calorimeter measures the energy change of a chemical reaction. A tutorial guide on how to calculate the heat of combustion. This type of calorimeter consists of. Bomb Calorimeter Chemistry.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Bomb Calorimeter Chemistry Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. So, for a constant volume, δe = q, and therefore, the bomb calorimeter measures the energy change of a chemical reaction. This type of calorimeter consists of a robust steel container (the “bomb”) that contains the reactants and is itself submerged in. Bomb Calorimeter Chemistry.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Bomb Calorimeter Chemistry So, for a constant volume, δe = q, and therefore, the bomb calorimeter measures the energy change of a chemical reaction. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. This lab demonstrates one of the most common. A bomb calorimeter is an instrument used to determine the heat emitted from. Bomb Calorimeter Chemistry.
From studylib.net
1b bomb calorimeter Bomb Calorimeter Chemistry Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. A bomb calorimeter is an instrument used to determine the heat emitted from a given quantity of biomass sample combustion and to calculate the hhv. Bomb Calorimeter Chemistry.
From www.studypool.com
SOLUTION Bomb calorimeter chemistry class 11, bomb calorimeter pdf, bomb calorimeter working Bomb Calorimeter Chemistry A bomb calorimeter is an instrument used to determine the heat emitted from a given quantity of biomass sample combustion and to calculate the hhv of that biomass fuel. Compare and contrast coffee cup calorimetry and bomb calorimetry. This type of calorimeter consists of a robust steel container (the “bomb”) that contains the reactants and is itself submerged in water. Bomb Calorimeter Chemistry.
From glossary.periodni.com
Calorimeter Chemistry Dictionary & Glossary Bomb Calorimeter Chemistry A bomb calorimeter is an instrument used to determine the heat emitted from a given quantity of biomass sample combustion and to calculate the hhv of that biomass fuel. Compare and contrast coffee cup calorimetry and bomb calorimetry. A tutorial guide on how to calculate the heat of combustion. This lab demonstrates one of the most common. Calculate heat, temperature. Bomb Calorimeter Chemistry.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Bomb Calorimeter Chemistry A bomb calorimeter is an instrument used to determine the heat emitted from a given quantity of biomass sample combustion and to calculate the hhv of that biomass fuel. So, for a constant volume, δe = q, and therefore, the bomb calorimeter measures the energy change of a chemical reaction. Describe a simple calorimeter and explain how it is employed. Bomb Calorimeter Chemistry.
From www.youtube.com
Bomb Calorimetry YouTube Bomb Calorimeter Chemistry Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Compare and contrast coffee cup calorimetry and bomb calorimetry. This type of calorimeter consists of a robust steel container (the “bomb”) that contains the reactants and is itself submerged in water (figure 5.17). A tutorial guide on how to calculate the heat. Bomb Calorimeter Chemistry.
From www.studypool.com
SOLUTION Bomb calorimeter explain with diagram and example? Studypool Bomb Calorimeter Chemistry This lab demonstrates one of the most common. So, for a constant volume, δe = q, and therefore, the bomb calorimeter measures the energy change of a chemical reaction. Compare and contrast coffee cup calorimetry and bomb calorimetry. This type of calorimeter consists of a robust steel container (the “bomb”) that contains the reactants and is itself submerged in water. Bomb Calorimeter Chemistry.
From www.studypool.com
SOLUTION Bomb calorimeter explain with diagram and example? Studypool Bomb Calorimeter Chemistry This type of calorimeter consists of a robust steel container (the “bomb”) that contains the reactants and is itself submerged in water (figure 5.17). A tutorial guide on how to calculate the heat of combustion. Compare and contrast coffee cup calorimetry and bomb calorimetry. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in. Bomb Calorimeter Chemistry.
From www.youtube.com
Bomb calorimeter class 11 chemistry Ch7 Lecture10B Chemistry ideas YouTube Bomb Calorimeter Chemistry Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. A bomb calorimeter is an instrument used to determine the heat emitted from a given quantity of biomass sample combustion and to calculate the hhv of that biomass fuel. Compare and contrast coffee cup calorimetry and bomb calorimetry. A tutorial guide on how to calculate the heat. Bomb Calorimeter Chemistry.
From www.slideserve.com
PPT Chemistry A Molecular Approach , 1 st Edition Nivaldo Tro PowerPoint Presentation ID Bomb Calorimeter Chemistry A tutorial guide on how to calculate the heat of combustion. This lab demonstrates one of the most common. Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. This type of calorimeter consists of a robust steel container (the “bomb”) that contains the reactants and is itself submerged in water (figure 5.17).. Bomb Calorimeter Chemistry.
From www.youtube.com
Physical Chemistry iBook Bomb Calorimetry YouTube Bomb Calorimeter Chemistry Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. A tutorial guide on how to calculate the heat of combustion. So, for a constant volume, δe = q, and therefore, the bomb calorimeter measures the energy change of a chemical reaction. This lab demonstrates one of the most common. Calculate heat, temperature change, and specific heat. Bomb Calorimeter Chemistry.
From www.youtube.com
Calorimetry, Bomb Calorimetry, Constant Pressure Calorimetry FULL Review for Exams YouTube Bomb Calorimeter Chemistry This lab demonstrates one of the most common. So, for a constant volume, δe = q, and therefore, the bomb calorimeter measures the energy change of a chemical reaction. A bomb calorimeter is an instrument used to determine the heat emitted from a given quantity of biomass sample combustion and to calculate the hhv of that biomass fuel. A tutorial. Bomb Calorimeter Chemistry.
From chemlab.truman.edu
Parr 1341 Bomb Calorimeter Chem Lab Bomb Calorimeter Chemistry A bomb calorimeter is an instrument used to determine the heat emitted from a given quantity of biomass sample combustion and to calculate the hhv of that biomass fuel. Compare and contrast coffee cup calorimetry and bomb calorimetry. Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. So, for a constant volume,. Bomb Calorimeter Chemistry.
From www.learner.org
The Energy in Chemical Reactions Thermodynamics and Enthalpy Annenberg Learner Bomb Calorimeter Chemistry So, for a constant volume, δe = q, and therefore, the bomb calorimeter measures the energy change of a chemical reaction. This lab demonstrates one of the most common. Compare and contrast coffee cup calorimetry and bomb calorimetry. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. Calculate heat, temperature change, and specific heat after thermal. Bomb Calorimeter Chemistry.
From www.slideserve.com
PPT TOPIC 8 THERMOCHEMISTRY PowerPoint Presentation, free download ID3087554 Bomb Calorimeter Chemistry A tutorial guide on how to calculate the heat of combustion. Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Compare and contrast coffee cup calorimetry and bomb calorimetry. This type of calorimeter consists. Bomb Calorimeter Chemistry.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Bomb Calorimeter Chemistry Compare and contrast coffee cup calorimetry and bomb calorimetry. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. A bomb calorimeter is an instrument used to determine the heat emitted from a given quantity of biomass sample combustion. Bomb Calorimeter Chemistry.
From www.shutterstock.com
Bomb Calorimeter Vector Illustration Labeled Educational Stock Vector (Royalty Free) 1607802400 Bomb Calorimeter Chemistry Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. A bomb calorimeter is an instrument used to determine the heat emitted from a given quantity of biomass sample combustion and to calculate the hhv of that biomass fuel. A tutorial guide on how to calculate the heat of combustion. So, for. Bomb Calorimeter Chemistry.
From www.pathwaystochemistry.com
Calorimetry Pathways to Chemistry Bomb Calorimeter Chemistry Compare and contrast coffee cup calorimetry and bomb calorimetry. Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. This type of calorimeter consists of a robust steel container (the “bomb”) that contains the reactants and is itself submerged in water (figure 5.17). So, for a constant volume, δe = q, and therefore,. Bomb Calorimeter Chemistry.
From www.youtube.com
Measuring Energy at Constant Volume Using a Bomb Calorimeter YouTube Bomb Calorimeter Chemistry Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. This type of calorimeter consists of a robust steel container (the “bomb”) that contains the reactants and is itself submerged in water (figure 5.17). Compare and contrast coffee cup calorimetry and bomb calorimetry. A tutorial guide on how to calculate the heat of. Bomb Calorimeter Chemistry.
From saylordotorg.github.io
Calorimetry Bomb Calorimeter Chemistry This type of calorimeter consists of a robust steel container (the “bomb”) that contains the reactants and is itself submerged in water (figure 5.17). Compare and contrast coffee cup calorimetry and bomb calorimetry. Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. So, for a constant volume, δe = q, and therefore,. Bomb Calorimeter Chemistry.
From www.slideserve.com
PPT Chapter 6 Thermochemistry PowerPoint Presentation, free download ID5118958 Bomb Calorimeter Chemistry Compare and contrast coffee cup calorimetry and bomb calorimetry. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. So, for a constant volume, δe = q, and therefore, the bomb calorimeter measures the energy change of a chemical reaction. This type of calorimeter consists of a robust steel container (the “bomb”). Bomb Calorimeter Chemistry.
From shaunmwilliams.com
Chapter 6 Presentation Bomb Calorimeter Chemistry Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. So, for a constant volume, δe = q, and therefore, the bomb calorimeter measures the energy change of a chemical reaction. A tutorial guide on how to calculate the heat of combustion. Calculate the molar heat of enthalpy for a reactions using. Bomb Calorimeter Chemistry.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Bomb Calorimeter Chemistry Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. A tutorial guide on how to calculate the heat of combustion. Compare and contrast coffee cup calorimetry and bomb calorimetry. This lab demonstrates one of. Bomb Calorimeter Chemistry.
From courses.lumenlearning.com
Calorimetry Chemistry Bomb Calorimeter Chemistry Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. So, for a constant volume, δe = q, and therefore, the bomb calorimeter measures the energy change of a chemical reaction. Compare and contrast coffee cup calorimetry and bomb calorimetry. Calculate the molar heat of enthalpy for a reactions using coffee cup. Bomb Calorimeter Chemistry.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Bomb Calorimeter Chemistry So, for a constant volume, δe = q, and therefore, the bomb calorimeter measures the energy change of a chemical reaction. Compare and contrast coffee cup calorimetry and bomb calorimetry. This lab demonstrates one of the most common. A bomb calorimeter is an instrument used to determine the heat emitted from a given quantity of biomass sample combustion and to. Bomb Calorimeter Chemistry.
From www.thoughtco.com
Calorimeter Definition in Chemistry Bomb Calorimeter Chemistry This type of calorimeter consists of a robust steel container (the “bomb”) that contains the reactants and is itself submerged in water (figure 5.17). Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. Describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. So, for a constant volume, δe. Bomb Calorimeter Chemistry.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID9276632 Bomb Calorimeter Chemistry A bomb calorimeter is an instrument used to determine the heat emitted from a given quantity of biomass sample combustion and to calculate the hhv of that biomass fuel. So, for a constant volume, δe = q, and therefore, the bomb calorimeter measures the energy change of a chemical reaction. A tutorial guide on how to calculate the heat of. Bomb Calorimeter Chemistry.