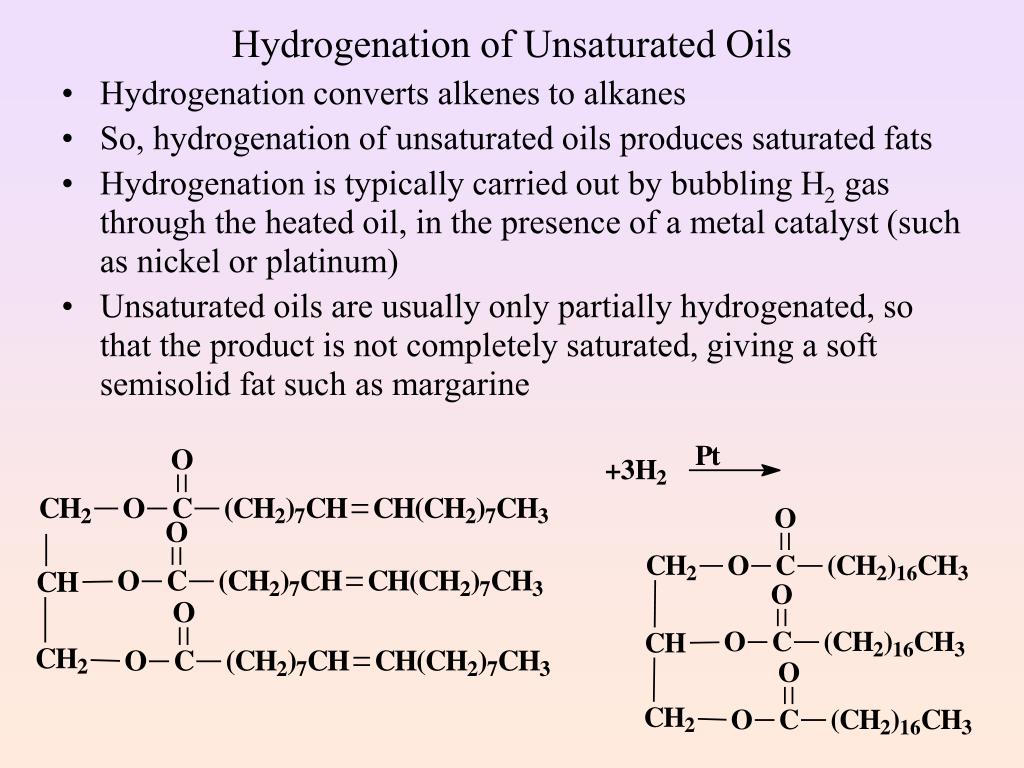
Hydrogenation Of Vegetable Oil Is An Example For . You can recognise the presence of this in foods because the ingredients. The melting point of a hydrogenated fat can be controlled by the degree of hydrogenation. A simple hydrogenation reaction is: Hydrogenation of vegetable oil increases the saturated fatty acid whereas decreases the unsaturated fatty acid (mathew and thangaraja,. But the many triglycerides it contains with varied fatty acid chains. Vegetable oils contain practically exclusively. The most unsaturated fatty acid groups are most easily hydrogenated and thus react first with the hydrogen if conditions are right. In the hydrogenation of vegetable oils and fatty acids, for example, the heat released, about 25 kcal per mole (105 kj/mol), is sufficient to. \[\ce{h_2c=ch_2 + h_2 \rightarrow ch_3ch_3}\] alkene plus hydrogen yields an alkane.
from www.slideserve.com
Vegetable oils contain practically exclusively. But the many triglycerides it contains with varied fatty acid chains. Hydrogenation of vegetable oil increases the saturated fatty acid whereas decreases the unsaturated fatty acid (mathew and thangaraja,. \[\ce{h_2c=ch_2 + h_2 \rightarrow ch_3ch_3}\] alkene plus hydrogen yields an alkane. A simple hydrogenation reaction is: The melting point of a hydrogenated fat can be controlled by the degree of hydrogenation. The most unsaturated fatty acid groups are most easily hydrogenated and thus react first with the hydrogen if conditions are right. In the hydrogenation of vegetable oils and fatty acids, for example, the heat released, about 25 kcal per mole (105 kj/mol), is sufficient to. You can recognise the presence of this in foods because the ingredients.
PPT Lipids PowerPoint Presentation, free download ID6534192
Hydrogenation Of Vegetable Oil Is An Example For But the many triglycerides it contains with varied fatty acid chains. You can recognise the presence of this in foods because the ingredients. Hydrogenation of vegetable oil increases the saturated fatty acid whereas decreases the unsaturated fatty acid (mathew and thangaraja,. But the many triglycerides it contains with varied fatty acid chains. Vegetable oils contain practically exclusively. In the hydrogenation of vegetable oils and fatty acids, for example, the heat released, about 25 kcal per mole (105 kj/mol), is sufficient to. The melting point of a hydrogenated fat can be controlled by the degree of hydrogenation. \[\ce{h_2c=ch_2 + h_2 \rightarrow ch_3ch_3}\] alkene plus hydrogen yields an alkane. A simple hydrogenation reaction is: The most unsaturated fatty acid groups are most easily hydrogenated and thus react first with the hydrogen if conditions are right.
From www.slideshare.net
Food Additives Hydrogenation Of Vegetable Oil Is An Example For Vegetable oils contain practically exclusively. In the hydrogenation of vegetable oils and fatty acids, for example, the heat released, about 25 kcal per mole (105 kj/mol), is sufficient to. The most unsaturated fatty acid groups are most easily hydrogenated and thus react first with the hydrogen if conditions are right. The melting point of a hydrogenated fat can be controlled. Hydrogenation Of Vegetable Oil Is An Example For.
From www.visionlearning.com
Lipids Biology Visionlearning Hydrogenation Of Vegetable Oil Is An Example For A simple hydrogenation reaction is: Hydrogenation of vegetable oil increases the saturated fatty acid whereas decreases the unsaturated fatty acid (mathew and thangaraja,. In the hydrogenation of vegetable oils and fatty acids, for example, the heat released, about 25 kcal per mole (105 kj/mol), is sufficient to. \[\ce{h_2c=ch_2 + h_2 \rightarrow ch_3ch_3}\] alkene plus hydrogen yields an alkane. The most. Hydrogenation Of Vegetable Oil Is An Example For.
From www.slideserve.com
PPT Friday 27 th April Vegetable Oils Learning Objectives PowerPoint Presentation ID1598175 Hydrogenation Of Vegetable Oil Is An Example For Hydrogenation of vegetable oil increases the saturated fatty acid whereas decreases the unsaturated fatty acid (mathew and thangaraja,. The most unsaturated fatty acid groups are most easily hydrogenated and thus react first with the hydrogen if conditions are right. Vegetable oils contain practically exclusively. The melting point of a hydrogenated fat can be controlled by the degree of hydrogenation. \[\ce{h_2c=ch_2. Hydrogenation Of Vegetable Oil Is An Example For.
From www.youtube.com
Hydrogenation Process of Vegetable Oil With Diagram II Why Oil are Partially Hydrogenated II L5 Hydrogenation Of Vegetable Oil Is An Example For But the many triglycerides it contains with varied fatty acid chains. The melting point of a hydrogenated fat can be controlled by the degree of hydrogenation. The most unsaturated fatty acid groups are most easily hydrogenated and thus react first with the hydrogen if conditions are right. In the hydrogenation of vegetable oils and fatty acids, for example, the heat. Hydrogenation Of Vegetable Oil Is An Example For.
From www.slideserve.com
PPT Alkenes PowerPoint Presentation, free download ID171306 Hydrogenation Of Vegetable Oil Is An Example For The melting point of a hydrogenated fat can be controlled by the degree of hydrogenation. \[\ce{h_2c=ch_2 + h_2 \rightarrow ch_3ch_3}\] alkene plus hydrogen yields an alkane. But the many triglycerides it contains with varied fatty acid chains. Vegetable oils contain practically exclusively. A simple hydrogenation reaction is: The most unsaturated fatty acid groups are most easily hydrogenated and thus react. Hydrogenation Of Vegetable Oil Is An Example For.
From slideplayer.com
Tuesday, 13 November 2018 Unsaturated Oils ppt download Hydrogenation Of Vegetable Oil Is An Example For The most unsaturated fatty acid groups are most easily hydrogenated and thus react first with the hydrogen if conditions are right. A simple hydrogenation reaction is: Vegetable oils contain practically exclusively. Hydrogenation of vegetable oil increases the saturated fatty acid whereas decreases the unsaturated fatty acid (mathew and thangaraja,. \[\ce{h_2c=ch_2 + h_2 \rightarrow ch_3ch_3}\] alkene plus hydrogen yields an alkane.. Hydrogenation Of Vegetable Oil Is An Example For.
From byjus.com
72.Hydrogenation of vegetable oilis what kind of reaction and what hydrogenation basically is Hydrogenation Of Vegetable Oil Is An Example For \[\ce{h_2c=ch_2 + h_2 \rightarrow ch_3ch_3}\] alkene plus hydrogen yields an alkane. The melting point of a hydrogenated fat can be controlled by the degree of hydrogenation. But the many triglycerides it contains with varied fatty acid chains. A simple hydrogenation reaction is: Hydrogenation of vegetable oil increases the saturated fatty acid whereas decreases the unsaturated fatty acid (mathew and thangaraja,.. Hydrogenation Of Vegetable Oil Is An Example For.
From www.researchgate.net
Composition of hydrogenated vegetable oils and vegetable oil... Download Table Hydrogenation Of Vegetable Oil Is An Example For In the hydrogenation of vegetable oils and fatty acids, for example, the heat released, about 25 kcal per mole (105 kj/mol), is sufficient to. Hydrogenation of vegetable oil increases the saturated fatty acid whereas decreases the unsaturated fatty acid (mathew and thangaraja,. The melting point of a hydrogenated fat can be controlled by the degree of hydrogenation. \[\ce{h_2c=ch_2 + h_2. Hydrogenation Of Vegetable Oil Is An Example For.
From www.slideshare.net
Hydrogenation of Edible Oils Hydrogenation Of Vegetable Oil Is An Example For \[\ce{h_2c=ch_2 + h_2 \rightarrow ch_3ch_3}\] alkene plus hydrogen yields an alkane. The melting point of a hydrogenated fat can be controlled by the degree of hydrogenation. The most unsaturated fatty acid groups are most easily hydrogenated and thus react first with the hydrogen if conditions are right. A simple hydrogenation reaction is: Vegetable oils contain practically exclusively. Hydrogenation of vegetable. Hydrogenation Of Vegetable Oil Is An Example For.
From www.slideserve.com
PPT Chemistry 2100 PowerPoint Presentation, free download ID6574979 Hydrogenation Of Vegetable Oil Is An Example For The most unsaturated fatty acid groups are most easily hydrogenated and thus react first with the hydrogen if conditions are right. Hydrogenation of vegetable oil increases the saturated fatty acid whereas decreases the unsaturated fatty acid (mathew and thangaraja,. You can recognise the presence of this in foods because the ingredients. The melting point of a hydrogenated fat can be. Hydrogenation Of Vegetable Oil Is An Example For.
From www.youtube.com
Changing of vegetable oil into ghee HydrogenationVanaspati GheeB.Sc, TET & M.Sc Hydrogenation Of Vegetable Oil Is An Example For In the hydrogenation of vegetable oils and fatty acids, for example, the heat released, about 25 kcal per mole (105 kj/mol), is sufficient to. The most unsaturated fatty acid groups are most easily hydrogenated and thus react first with the hydrogen if conditions are right. You can recognise the presence of this in foods because the ingredients. The melting point. Hydrogenation Of Vegetable Oil Is An Example For.
From www.scribd.com
Hydrogenation of Vegetable Oil To Margarine PDF Hydrogenation Vegetable Oil Hydrogenation Of Vegetable Oil Is An Example For Hydrogenation of vegetable oil increases the saturated fatty acid whereas decreases the unsaturated fatty acid (mathew and thangaraja,. A simple hydrogenation reaction is: The melting point of a hydrogenated fat can be controlled by the degree of hydrogenation. In the hydrogenation of vegetable oils and fatty acids, for example, the heat released, about 25 kcal per mole (105 kj/mol), is. Hydrogenation Of Vegetable Oil Is An Example For.
From www.slideserve.com
PPT The Skinny on Trans Fats PowerPoint Presentation, free download ID14253 Hydrogenation Of Vegetable Oil Is An Example For But the many triglycerides it contains with varied fatty acid chains. You can recognise the presence of this in foods because the ingredients. \[\ce{h_2c=ch_2 + h_2 \rightarrow ch_3ch_3}\] alkene plus hydrogen yields an alkane. Hydrogenation of vegetable oil increases the saturated fatty acid whereas decreases the unsaturated fatty acid (mathew and thangaraja,. In the hydrogenation of vegetable oils and fatty. Hydrogenation Of Vegetable Oil Is An Example For.
From www.bioanalyt.com
How is edible oil refined? Fortified Food Vehicles Series Bioanalyt Hydrogenation Of Vegetable Oil Is An Example For The most unsaturated fatty acid groups are most easily hydrogenated and thus react first with the hydrogen if conditions are right. In the hydrogenation of vegetable oils and fatty acids, for example, the heat released, about 25 kcal per mole (105 kj/mol), is sufficient to. The melting point of a hydrogenated fat can be controlled by the degree of hydrogenation.. Hydrogenation Of Vegetable Oil Is An Example For.
From ar.inspiredpencil.com
Hydrogenated Oil Structure Hydrogenation Of Vegetable Oil Is An Example For But the many triglycerides it contains with varied fatty acid chains. You can recognise the presence of this in foods because the ingredients. Hydrogenation of vegetable oil increases the saturated fatty acid whereas decreases the unsaturated fatty acid (mathew and thangaraja,. A simple hydrogenation reaction is: Vegetable oils contain practically exclusively. The melting point of a hydrogenated fat can be. Hydrogenation Of Vegetable Oil Is An Example For.
From learncanyon.com
Hydrogenated Vegetable Oil Learn Canyon Hydrogenation Of Vegetable Oil Is An Example For The most unsaturated fatty acid groups are most easily hydrogenated and thus react first with the hydrogen if conditions are right. A simple hydrogenation reaction is: Vegetable oils contain practically exclusively. But the many triglycerides it contains with varied fatty acid chains. In the hydrogenation of vegetable oils and fatty acids, for example, the heat released, about 25 kcal per. Hydrogenation Of Vegetable Oil Is An Example For.
From www.slideshare.net
Hydrogenation of Edible Oils Hydrogenation Of Vegetable Oil Is An Example For A simple hydrogenation reaction is: The most unsaturated fatty acid groups are most easily hydrogenated and thus react first with the hydrogen if conditions are right. In the hydrogenation of vegetable oils and fatty acids, for example, the heat released, about 25 kcal per mole (105 kj/mol), is sufficient to. But the many triglycerides it contains with varied fatty acid. Hydrogenation Of Vegetable Oil Is An Example For.
From www.teachoo.com
[Carbon Class 10] Hydrogenation of oils Definition, Reaction Hydrogenation Of Vegetable Oil Is An Example For You can recognise the presence of this in foods because the ingredients. The melting point of a hydrogenated fat can be controlled by the degree of hydrogenation. A simple hydrogenation reaction is: In the hydrogenation of vegetable oils and fatty acids, for example, the heat released, about 25 kcal per mole (105 kj/mol), is sufficient to. But the many triglycerides. Hydrogenation Of Vegetable Oil Is An Example For.
From www.studocu.com
PFDHydrogenation of Oils Hydrogenation of vegetable oils Hydrogenation process is used to Hydrogenation Of Vegetable Oil Is An Example For The melting point of a hydrogenated fat can be controlled by the degree of hydrogenation. In the hydrogenation of vegetable oils and fatty acids, for example, the heat released, about 25 kcal per mole (105 kj/mol), is sufficient to. You can recognise the presence of this in foods because the ingredients. But the many triglycerides it contains with varied fatty. Hydrogenation Of Vegetable Oil Is An Example For.
From www.slideserve.com
PPT Partial Hydrogenation of Vegetable Oil using Membrane Reactor Technology PowerPoint Hydrogenation Of Vegetable Oil Is An Example For You can recognise the presence of this in foods because the ingredients. Vegetable oils contain practically exclusively. The most unsaturated fatty acid groups are most easily hydrogenated and thus react first with the hydrogen if conditions are right. Hydrogenation of vegetable oil increases the saturated fatty acid whereas decreases the unsaturated fatty acid (mathew and thangaraja,. \[\ce{h_2c=ch_2 + h_2 \rightarrow. Hydrogenation Of Vegetable Oil Is An Example For.
From www.slideserve.com
PPT Chapter 13 Alkanes, Alkynes, and Aromatic Compounds PowerPoint Presentation ID6072810 Hydrogenation Of Vegetable Oil Is An Example For \[\ce{h_2c=ch_2 + h_2 \rightarrow ch_3ch_3}\] alkene plus hydrogen yields an alkane. You can recognise the presence of this in foods because the ingredients. But the many triglycerides it contains with varied fatty acid chains. The most unsaturated fatty acid groups are most easily hydrogenated and thus react first with the hydrogen if conditions are right. Hydrogenation of vegetable oil increases. Hydrogenation Of Vegetable Oil Is An Example For.
From www.youtube.com
How To Harden Vegetable Oils Through Hydrogenation Organic Chemistry Chemistry FuseSchool Hydrogenation Of Vegetable Oil Is An Example For The melting point of a hydrogenated fat can be controlled by the degree of hydrogenation. In the hydrogenation of vegetable oils and fatty acids, for example, the heat released, about 25 kcal per mole (105 kj/mol), is sufficient to. A simple hydrogenation reaction is: The most unsaturated fatty acid groups are most easily hydrogenated and thus react first with the. Hydrogenation Of Vegetable Oil Is An Example For.
From www.researchgate.net
(PDF) Hydrogenated Vegetable Oil Is it Healthy? Hydrogenation Of Vegetable Oil Is An Example For Vegetable oils contain practically exclusively. But the many triglycerides it contains with varied fatty acid chains. The most unsaturated fatty acid groups are most easily hydrogenated and thus react first with the hydrogen if conditions are right. \[\ce{h_2c=ch_2 + h_2 \rightarrow ch_3ch_3}\] alkene plus hydrogen yields an alkane. The melting point of a hydrogenated fat can be controlled by the. Hydrogenation Of Vegetable Oil Is An Example For.
From www.azom.com
What is Hydrogenation? Hydrogenation Of Vegetable Oil Is An Example For Vegetable oils contain practically exclusively. The most unsaturated fatty acid groups are most easily hydrogenated and thus react first with the hydrogen if conditions are right. A simple hydrogenation reaction is: \[\ce{h_2c=ch_2 + h_2 \rightarrow ch_3ch_3}\] alkene plus hydrogen yields an alkane. In the hydrogenation of vegetable oils and fatty acids, for example, the heat released, about 25 kcal per. Hydrogenation Of Vegetable Oil Is An Example For.
From www.scribd.com
Hydrogenation of Vegetable Oil PDF Hydrogenation Vegetable Oil Hydrogenation Of Vegetable Oil Is An Example For The melting point of a hydrogenated fat can be controlled by the degree of hydrogenation. The most unsaturated fatty acid groups are most easily hydrogenated and thus react first with the hydrogen if conditions are right. You can recognise the presence of this in foods because the ingredients. A simple hydrogenation reaction is: In the hydrogenation of vegetable oils and. Hydrogenation Of Vegetable Oil Is An Example For.
From www.slideserve.com
PPT Lipids PowerPoint Presentation, free download ID6534192 Hydrogenation Of Vegetable Oil Is An Example For You can recognise the presence of this in foods because the ingredients. But the many triglycerides it contains with varied fatty acid chains. The melting point of a hydrogenated fat can be controlled by the degree of hydrogenation. In the hydrogenation of vegetable oils and fatty acids, for example, the heat released, about 25 kcal per mole (105 kj/mol), is. Hydrogenation Of Vegetable Oil Is An Example For.
From www.youtube.com
B.10 Hydrogenation of oils (HL) YouTube Hydrogenation Of Vegetable Oil Is An Example For \[\ce{h_2c=ch_2 + h_2 \rightarrow ch_3ch_3}\] alkene plus hydrogen yields an alkane. In the hydrogenation of vegetable oils and fatty acids, for example, the heat released, about 25 kcal per mole (105 kj/mol), is sufficient to. But the many triglycerides it contains with varied fatty acid chains. Hydrogenation of vegetable oil increases the saturated fatty acid whereas decreases the unsaturated fatty. Hydrogenation Of Vegetable Oil Is An Example For.
From www.weekand.com
Facts about Hydrogenated Vegetable Oil Hydrogenation Of Vegetable Oil Is An Example For The most unsaturated fatty acid groups are most easily hydrogenated and thus react first with the hydrogen if conditions are right. \[\ce{h_2c=ch_2 + h_2 \rightarrow ch_3ch_3}\] alkene plus hydrogen yields an alkane. Hydrogenation of vegetable oil increases the saturated fatty acid whereas decreases the unsaturated fatty acid (mathew and thangaraja,. Vegetable oils contain practically exclusively. But the many triglycerides it. Hydrogenation Of Vegetable Oil Is An Example For.
From courses.lumenlearning.com
Hydrogen Boundless Chemistry Hydrogenation Of Vegetable Oil Is An Example For In the hydrogenation of vegetable oils and fatty acids, for example, the heat released, about 25 kcal per mole (105 kj/mol), is sufficient to. Vegetable oils contain practically exclusively. \[\ce{h_2c=ch_2 + h_2 \rightarrow ch_3ch_3}\] alkene plus hydrogen yields an alkane. But the many triglycerides it contains with varied fatty acid chains. A simple hydrogenation reaction is: You can recognise the. Hydrogenation Of Vegetable Oil Is An Example For.
From www.researchgate.net
(PDF) Electrocatalytic Hydrogenation of Vegetable Oil Hydrogenation Of Vegetable Oil Is An Example For Vegetable oils contain practically exclusively. A simple hydrogenation reaction is: In the hydrogenation of vegetable oils and fatty acids, for example, the heat released, about 25 kcal per mole (105 kj/mol), is sufficient to. \[\ce{h_2c=ch_2 + h_2 \rightarrow ch_3ch_3}\] alkene plus hydrogen yields an alkane. Hydrogenation of vegetable oil increases the saturated fatty acid whereas decreases the unsaturated fatty acid. Hydrogenation Of Vegetable Oil Is An Example For.
From www.youtube.com
Hydrogenation of oils to form margarine YouTube Hydrogenation Of Vegetable Oil Is An Example For The melting point of a hydrogenated fat can be controlled by the degree of hydrogenation. You can recognise the presence of this in foods because the ingredients. The most unsaturated fatty acid groups are most easily hydrogenated and thus react first with the hydrogen if conditions are right. In the hydrogenation of vegetable oils and fatty acids, for example, the. Hydrogenation Of Vegetable Oil Is An Example For.
From www.youtube.com
HYDROGENATION OF VEGETABLE OIL YouTube Hydrogenation Of Vegetable Oil Is An Example For A simple hydrogenation reaction is: Hydrogenation of vegetable oil increases the saturated fatty acid whereas decreases the unsaturated fatty acid (mathew and thangaraja,. You can recognise the presence of this in foods because the ingredients. But the many triglycerides it contains with varied fatty acid chains. The melting point of a hydrogenated fat can be controlled by the degree of. Hydrogenation Of Vegetable Oil Is An Example For.
From www.youtube.com
What is the hydrogenation of vegetable oils? 8 HYDROGEN CHEMISTRY ICSE Doubtnut YouTube Hydrogenation Of Vegetable Oil Is An Example For \[\ce{h_2c=ch_2 + h_2 \rightarrow ch_3ch_3}\] alkene plus hydrogen yields an alkane. You can recognise the presence of this in foods because the ingredients. A simple hydrogenation reaction is: Vegetable oils contain practically exclusively. Hydrogenation of vegetable oil increases the saturated fatty acid whereas decreases the unsaturated fatty acid (mathew and thangaraja,. The most unsaturated fatty acid groups are most easily. Hydrogenation Of Vegetable Oil Is An Example For.
From www.teachoo.com
[Carbon Class 10] Hydrogenation of oils Definition, Reaction Hydrogenation Of Vegetable Oil Is An Example For You can recognise the presence of this in foods because the ingredients. The most unsaturated fatty acid groups are most easily hydrogenated and thus react first with the hydrogen if conditions are right. The melting point of a hydrogenated fat can be controlled by the degree of hydrogenation. In the hydrogenation of vegetable oils and fatty acids, for example, the. Hydrogenation Of Vegetable Oil Is An Example For.
From www.slideshare.net
Hydrogenation of Edible Oils Hydrogenation Of Vegetable Oil Is An Example For You can recognise the presence of this in foods because the ingredients. Vegetable oils contain practically exclusively. \[\ce{h_2c=ch_2 + h_2 \rightarrow ch_3ch_3}\] alkene plus hydrogen yields an alkane. Hydrogenation of vegetable oil increases the saturated fatty acid whereas decreases the unsaturated fatty acid (mathew and thangaraja,. But the many triglycerides it contains with varied fatty acid chains. The most unsaturated. Hydrogenation Of Vegetable Oil Is An Example For.