Partial Charges Of Water . The positively charged sodium ions in the crystal attract the oxygen end of the water molecules because they are partially negative. Explore the attractions and properties of polar water. The atom with the greater electronegativity acquires a partial negative charge, while the atom with the lesser. Water is a molecular compound consisting of polar molecules that have a bent shape. Water is attracted to the sodium chloride crystal because water is polar and has both a positive and a negative end. Water is a polar molecule with partial positive and negative charges on its atoms. Partial atomic charges provide an intuitive and efficient way to describe the charge distribution and the resulting intermolecular electrostatic. Learn how water molecules are polar due to unequal sharing of electrons between oxygen and hydrogen atoms. Learn how water's polarity affects its interactions with other polar. The oxygen atom acquires a partial negative charge,.
from www.chegg.com
The oxygen atom acquires a partial negative charge,. Water is a molecular compound consisting of polar molecules that have a bent shape. Partial atomic charges provide an intuitive and efficient way to describe the charge distribution and the resulting intermolecular electrostatic. Water is a polar molecule with partial positive and negative charges on its atoms. The atom with the greater electronegativity acquires a partial negative charge, while the atom with the lesser. Water is attracted to the sodium chloride crystal because water is polar and has both a positive and a negative end. Learn how water's polarity affects its interactions with other polar. Explore the attractions and properties of polar water. The positively charged sodium ions in the crystal attract the oxygen end of the water molecules because they are partially negative. Learn how water molecules are polar due to unequal sharing of electrons between oxygen and hydrogen atoms.
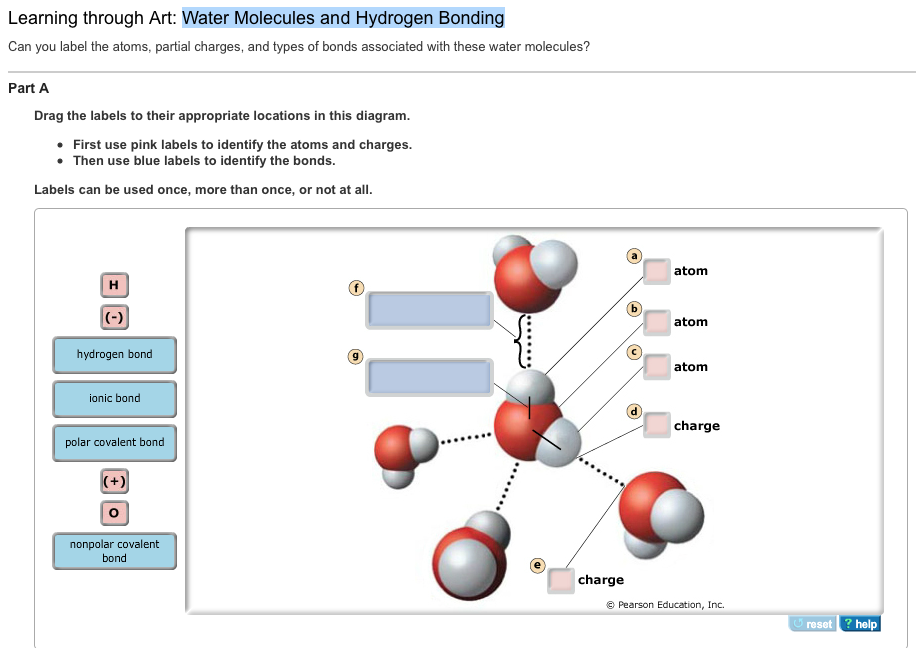
Solved Can you label the atoms, partial charges, and types
Partial Charges Of Water Explore the attractions and properties of polar water. Learn how water molecules are polar due to unequal sharing of electrons between oxygen and hydrogen atoms. The atom with the greater electronegativity acquires a partial negative charge, while the atom with the lesser. The oxygen atom acquires a partial negative charge,. Explore the attractions and properties of polar water. The positively charged sodium ions in the crystal attract the oxygen end of the water molecules because they are partially negative. Learn how water's polarity affects its interactions with other polar. Water is a polar molecule with partial positive and negative charges on its atoms. Water is attracted to the sodium chloride crystal because water is polar and has both a positive and a negative end. Partial atomic charges provide an intuitive and efficient way to describe the charge distribution and the resulting intermolecular electrostatic. Water is a molecular compound consisting of polar molecules that have a bent shape.
From www.coursehero.com
[Solved] . a) Draw the structure of a water molecule and show the partial... Course Hero Partial Charges Of Water Partial atomic charges provide an intuitive and efficient way to describe the charge distribution and the resulting intermolecular electrostatic. Water is a molecular compound consisting of polar molecules that have a bent shape. Learn how water molecules are polar due to unequal sharing of electrons between oxygen and hydrogen atoms. The positively charged sodium ions in the crystal attract the. Partial Charges Of Water.
From www.chegg.com
Solved Can you label the atoms, partial charges, and types Partial Charges Of Water The oxygen atom acquires a partial negative charge,. Explore the attractions and properties of polar water. Water is a molecular compound consisting of polar molecules that have a bent shape. Learn how water's polarity affects its interactions with other polar. The positively charged sodium ions in the crystal attract the oxygen end of the water molecules because they are partially. Partial Charges Of Water.
From bio1151.nicerweb.com
water_molecule.html 03_02WaterMolecules_L.jpg Partial Charges Of Water Explore the attractions and properties of polar water. Learn how water's polarity affects its interactions with other polar. Water is a molecular compound consisting of polar molecules that have a bent shape. The oxygen atom acquires a partial negative charge,. The positively charged sodium ions in the crystal attract the oxygen end of the water molecules because they are partially. Partial Charges Of Water.
From byjus.com
why oxygen in water has two partial negative charges? Partial Charges Of Water Partial atomic charges provide an intuitive and efficient way to describe the charge distribution and the resulting intermolecular electrostatic. The atom with the greater electronegativity acquires a partial negative charge, while the atom with the lesser. Water is attracted to the sodium chloride crystal because water is polar and has both a positive and a negative end. Water is a. Partial Charges Of Water.
From www.researchgate.net
Chemical structure and partial charges of (a) water, (b) decane, and... Download Scientific Partial Charges Of Water Learn how water molecules are polar due to unequal sharing of electrons between oxygen and hydrogen atoms. The oxygen atom acquires a partial negative charge,. Explore the attractions and properties of polar water. Water is a molecular compound consisting of polar molecules that have a bent shape. Water is a polar molecule with partial positive and negative charges on its. Partial Charges Of Water.
From www.clipartkey.com
H2o Chemical Bond Structure Of Water With Partial Charges , Free Transparent Clipart ClipartKey Partial Charges Of Water Water is a polar molecule with partial positive and negative charges on its atoms. Water is attracted to the sodium chloride crystal because water is polar and has both a positive and a negative end. Learn how water's polarity affects its interactions with other polar. The atom with the greater electronegativity acquires a partial negative charge, while the atom with. Partial Charges Of Water.
From thebiologyprimer.com
Atoms & Molecules echapter — The Biology Primer Partial Charges Of Water Explore the attractions and properties of polar water. Partial atomic charges provide an intuitive and efficient way to describe the charge distribution and the resulting intermolecular electrostatic. Water is attracted to the sodium chloride crystal because water is polar and has both a positive and a negative end. Learn how water's polarity affects its interactions with other polar. The oxygen. Partial Charges Of Water.
From socratic.org
What are the strongest bonds in water? Socratic Partial Charges Of Water Water is a molecular compound consisting of polar molecules that have a bent shape. Learn how water's polarity affects its interactions with other polar. Partial atomic charges provide an intuitive and efficient way to describe the charge distribution and the resulting intermolecular electrostatic. The atom with the greater electronegativity acquires a partial negative charge, while the atom with the lesser.. Partial Charges Of Water.
From biostudizz.weebly.com
WATER CHEMISTRY to Bio Stud... Partial Charges Of Water The atom with the greater electronegativity acquires a partial negative charge, while the atom with the lesser. Learn how water molecules are polar due to unequal sharing of electrons between oxygen and hydrogen atoms. Water is a polar molecule with partial positive and negative charges on its atoms. The oxygen atom acquires a partial negative charge,. Water is a molecular. Partial Charges Of Water.
From www.expii.com
Hydrogen Bonds — Overview & Examples Expii Partial Charges Of Water Learn how water molecules are polar due to unequal sharing of electrons between oxygen and hydrogen atoms. Explore the attractions and properties of polar water. The positively charged sodium ions in the crystal attract the oxygen end of the water molecules because they are partially negative. Water is attracted to the sodium chloride crystal because water is polar and has. Partial Charges Of Water.
From drawingideas.netlify.app
Draw A Water Molecule And Label The Partial Charges On The Molecule Partial Charges Of Water Partial atomic charges provide an intuitive and efficient way to describe the charge distribution and the resulting intermolecular electrostatic. Water is a polar molecule with partial positive and negative charges on its atoms. The oxygen atom acquires a partial negative charge,. Water is attracted to the sodium chloride crystal because water is polar and has both a positive and a. Partial Charges Of Water.
From courses.lumenlearning.com
The Structure and Properties of Water Introduction to Chemistry Partial Charges Of Water Learn how water's polarity affects its interactions with other polar. The atom with the greater electronegativity acquires a partial negative charge, while the atom with the lesser. Water is a polar molecule with partial positive and negative charges on its atoms. Learn how water molecules are polar due to unequal sharing of electrons between oxygen and hydrogen atoms. Water is. Partial Charges Of Water.
From blog.iglcoatings.com
The Science of Hydrophobicity IGL Coatings Blog Partial Charges Of Water Water is attracted to the sodium chloride crystal because water is polar and has both a positive and a negative end. Water is a molecular compound consisting of polar molecules that have a bent shape. Water is a polar molecule with partial positive and negative charges on its atoms. The atom with the greater electronegativity acquires a partial negative charge,. Partial Charges Of Water.
From galvinconanstuart.blogspot.com
Electron Distribution Diagram Of Water General Wiring Diagram Partial Charges Of Water The oxygen atom acquires a partial negative charge,. The atom with the greater electronegativity acquires a partial negative charge, while the atom with the lesser. Water is a polar molecule with partial positive and negative charges on its atoms. Learn how water's polarity affects its interactions with other polar. Water is attracted to the sodium chloride crystal because water is. Partial Charges Of Water.
From www.slideserve.com
PPT Structure and Properties of Water PowerPoint Presentation, free download ID80386 Partial Charges Of Water The atom with the greater electronegativity acquires a partial negative charge, while the atom with the lesser. Water is a polar molecule with partial positive and negative charges on its atoms. The oxygen atom acquires a partial negative charge,. Partial atomic charges provide an intuitive and efficient way to describe the charge distribution and the resulting intermolecular electrostatic. Explore the. Partial Charges Of Water.
From www.slideserve.com
PPT Solubility and Net Ionic Equations PowerPoint Presentation, free download ID3105334 Partial Charges Of Water Explore the attractions and properties of polar water. Water is a polar molecule with partial positive and negative charges on its atoms. Partial atomic charges provide an intuitive and efficient way to describe the charge distribution and the resulting intermolecular electrostatic. The positively charged sodium ions in the crystal attract the oxygen end of the water molecules because they are. Partial Charges Of Water.
From neurotext.library.stonybrook.edu
Cellular Neurophysiology Partial Charges Of Water The positively charged sodium ions in the crystal attract the oxygen end of the water molecules because they are partially negative. Water is a polar molecule with partial positive and negative charges on its atoms. Learn how water's polarity affects its interactions with other polar. Explore the attractions and properties of polar water. The atom with the greater electronegativity acquires. Partial Charges Of Water.
From drawingideas.netlify.app
Draw A Water Molecule And Label The Partial Charges On The Molecule Partial Charges Of Water Explore the attractions and properties of polar water. Learn how water molecules are polar due to unequal sharing of electrons between oxygen and hydrogen atoms. Water is attracted to the sodium chloride crystal because water is polar and has both a positive and a negative end. The oxygen atom acquires a partial negative charge,. The atom with the greater electronegativity. Partial Charges Of Water.
From www.numerade.com
SOLVED 5. a) Draw a water molecule and identify the partial charges on the water molecules. b Partial Charges Of Water Learn how water molecules are polar due to unequal sharing of electrons between oxygen and hydrogen atoms. Partial atomic charges provide an intuitive and efficient way to describe the charge distribution and the resulting intermolecular electrostatic. Water is a polar molecule with partial positive and negative charges on its atoms. The atom with the greater electronegativity acquires a partial negative. Partial Charges Of Water.
From brainly.com
Can you label the atoms, partial charges, and types of bonds associated with these water Partial Charges Of Water Partial atomic charges provide an intuitive and efficient way to describe the charge distribution and the resulting intermolecular electrostatic. Water is a polar molecule with partial positive and negative charges on its atoms. The atom with the greater electronegativity acquires a partial negative charge, while the atom with the lesser. Learn how water's polarity affects its interactions with other polar.. Partial Charges Of Water.
From www.slideserve.com
PPT Water Chemistry & Properties of Water PowerPoint Presentation ID6877014 Partial Charges Of Water Partial atomic charges provide an intuitive and efficient way to describe the charge distribution and the resulting intermolecular electrostatic. Explore the attractions and properties of polar water. Water is a molecular compound consisting of polar molecules that have a bent shape. Learn how water's polarity affects its interactions with other polar. The positively charged sodium ions in the crystal attract. Partial Charges Of Water.
From istudy.pk
WATER IN PLANT LIFE Study Solutions Partial Charges Of Water Water is a molecular compound consisting of polar molecules that have a bent shape. Learn how water molecules are polar due to unequal sharing of electrons between oxygen and hydrogen atoms. Water is a polar molecule with partial positive and negative charges on its atoms. Water is attracted to the sodium chloride crystal because water is polar and has both. Partial Charges Of Water.
From www.chegg.com
Solved Water is a polar molecule, meaning it carries partial Partial Charges Of Water Learn how water's polarity affects its interactions with other polar. Explore the attractions and properties of polar water. Water is a polar molecule with partial positive and negative charges on its atoms. The atom with the greater electronegativity acquires a partial negative charge, while the atom with the lesser. Learn how water molecules are polar due to unequal sharing of. Partial Charges Of Water.
From ar.inspiredpencil.com
Water Molecule With Charges Partial Charges Of Water Learn how water molecules are polar due to unequal sharing of electrons between oxygen and hydrogen atoms. Partial atomic charges provide an intuitive and efficient way to describe the charge distribution and the resulting intermolecular electrostatic. The positively charged sodium ions in the crystal attract the oxygen end of the water molecules because they are partially negative. Water is a. Partial Charges Of Water.
From www.numerade.com
SOLVED Vhich shows the Lewis structure of water with the correct partial charges &nd nonbonding Partial Charges Of Water The atom with the greater electronegativity acquires a partial negative charge, while the atom with the lesser. Water is a polar molecule with partial positive and negative charges on its atoms. Water is attracted to the sodium chloride crystal because water is polar and has both a positive and a negative end. Learn how water molecules are polar due to. Partial Charges Of Water.
From webmis.highland.cc.il.us
Aqueous Solutions Partial Charges Of Water Partial atomic charges provide an intuitive and efficient way to describe the charge distribution and the resulting intermolecular electrostatic. Water is attracted to the sodium chloride crystal because water is polar and has both a positive and a negative end. The atom with the greater electronegativity acquires a partial negative charge, while the atom with the lesser. Explore the attractions. Partial Charges Of Water.
From ar.inspiredpencil.com
Water Molecule With Charges Partial Charges Of Water The oxygen atom acquires a partial negative charge,. Water is a molecular compound consisting of polar molecules that have a bent shape. Learn how water's polarity affects its interactions with other polar. Partial atomic charges provide an intuitive and efficient way to describe the charge distribution and the resulting intermolecular electrostatic. The positively charged sodium ions in the crystal attract. Partial Charges Of Water.
From www.chegg.com
Solved Water is a polar molecule, meaning it carries partial Partial Charges Of Water Water is a molecular compound consisting of polar molecules that have a bent shape. Learn how water molecules are polar due to unequal sharing of electrons between oxygen and hydrogen atoms. The atom with the greater electronegativity acquires a partial negative charge, while the atom with the lesser. The positively charged sodium ions in the crystal attract the oxygen end. Partial Charges Of Water.
From chem.libretexts.org
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts Partial Charges Of Water The oxygen atom acquires a partial negative charge,. The positively charged sodium ions in the crystal attract the oxygen end of the water molecules because they are partially negative. Water is a molecular compound consisting of polar molecules that have a bent shape. Water is attracted to the sodium chloride crystal because water is polar and has both a positive. Partial Charges Of Water.
From www.coursehero.com
[Solved] Try this example with water first Water, H20 Partial charges H... Course Hero Partial Charges Of Water The atom with the greater electronegativity acquires a partial negative charge, while the atom with the lesser. The oxygen atom acquires a partial negative charge,. Partial atomic charges provide an intuitive and efficient way to describe the charge distribution and the resulting intermolecular electrostatic. Water is a molecular compound consisting of polar molecules that have a bent shape. The positively. Partial Charges Of Water.
From www.slideserve.com
PPT Polarity of water PowerPoint Presentation, free download ID2610682 Partial Charges Of Water Partial atomic charges provide an intuitive and efficient way to describe the charge distribution and the resulting intermolecular electrostatic. Water is a molecular compound consisting of polar molecules that have a bent shape. Water is a polar molecule with partial positive and negative charges on its atoms. Water is attracted to the sodium chloride crystal because water is polar and. Partial Charges Of Water.
From www.researchgate.net
Schematic diagram of the water molecules transports in AQP1. (a) How... Download Scientific Partial Charges Of Water Water is a molecular compound consisting of polar molecules that have a bent shape. Water is a polar molecule with partial positive and negative charges on its atoms. The positively charged sodium ions in the crystal attract the oxygen end of the water molecules because they are partially negative. Learn how water's polarity affects its interactions with other polar. Partial. Partial Charges Of Water.
From thebiologs.blogspot.com
The BioLogs CAPE 1 Water Introduction to it's BIOchemistry Partial Charges Of Water Water is attracted to the sodium chloride crystal because water is polar and has both a positive and a negative end. Explore the attractions and properties of polar water. Water is a polar molecule with partial positive and negative charges on its atoms. Partial atomic charges provide an intuitive and efficient way to describe the charge distribution and the resulting. Partial Charges Of Water.
From alevelbiology.co.uk
Water Structure And Properties Molecule & Physical Properties A Level Partial Charges Of Water The atom with the greater electronegativity acquires a partial negative charge, while the atom with the lesser. Learn how water molecules are polar due to unequal sharing of electrons between oxygen and hydrogen atoms. The oxygen atom acquires a partial negative charge,. Learn how water's polarity affects its interactions with other polar. Water is a polar molecule with partial positive. Partial Charges Of Water.
From www.chegg.com
Solved Label the following diagram of water molecules, Partial Charges Of Water Water is a molecular compound consisting of polar molecules that have a bent shape. The positively charged sodium ions in the crystal attract the oxygen end of the water molecules because they are partially negative. Partial atomic charges provide an intuitive and efficient way to describe the charge distribution and the resulting intermolecular electrostatic. Learn how water molecules are polar. Partial Charges Of Water.