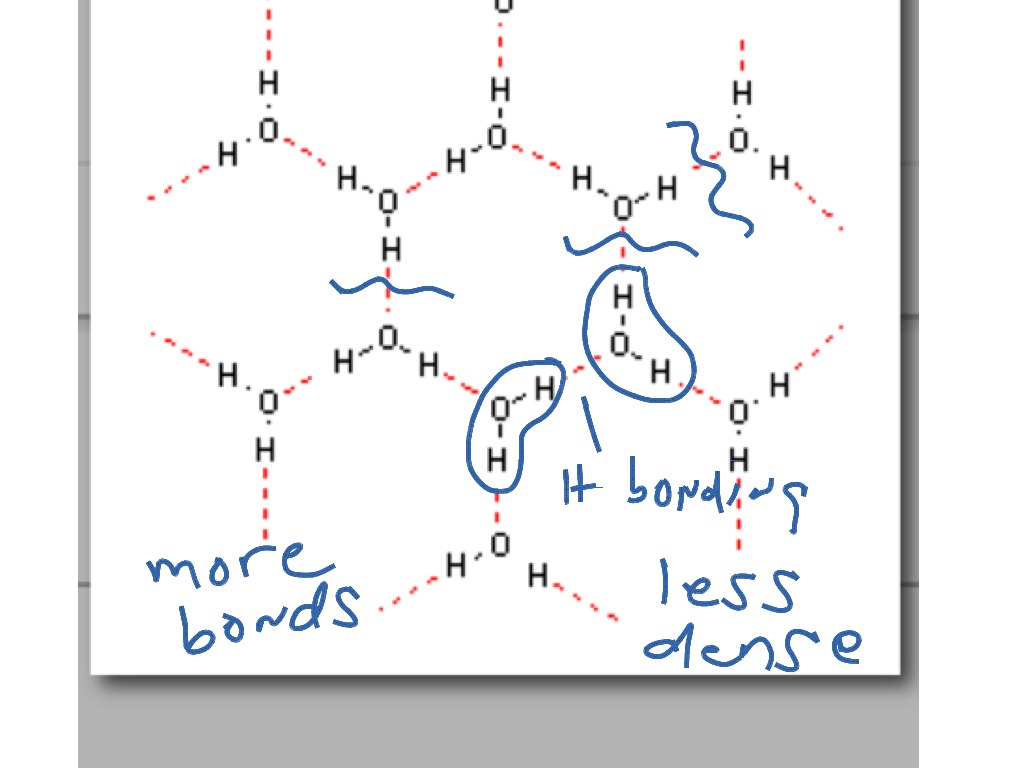
Why Does Ice Float Molecules . Learn the reason behind the peculiar behavior of ice cubes floating in water, which is due to the decrease in density of water molecules as they turn into ice. Ice floats because it is less dense than liquid water due to hydrogen bonding. Learn how ice's unique molecular structure and the anomalous expansion of water lead to its lower density and buoyancy. Why does ice float on water? Learn how this phenomenon affects lakes, rivers and fish, and how heavy water ice sinks in. Learn why ice floats on water and how the structure of ice differs from liquid water. Discover the implications of ice floating on. Ice has a hexagonal framework with open spaces that make it less dense than liquid water. Water molecules form hydrogen bonds when an oxygen atom (red) in one attracts a hydrogen atom (grey) in another (top). The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms.
from www.showme.com
Ice has a hexagonal framework with open spaces that make it less dense than liquid water. Ice floats because it is less dense than liquid water due to hydrogen bonding. Learn how ice's unique molecular structure and the anomalous expansion of water lead to its lower density and buoyancy. Discover the implications of ice floating on. Why does ice float on water? Learn how this phenomenon affects lakes, rivers and fish, and how heavy water ice sinks in. The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. Learn the reason behind the peculiar behavior of ice cubes floating in water, which is due to the decrease in density of water molecules as they turn into ice. Water molecules form hydrogen bonds when an oxygen atom (red) in one attracts a hydrogen atom (grey) in another (top). Learn why ice floats on water and how the structure of ice differs from liquid water.
Why does ice float? ShowMe
Why Does Ice Float Molecules The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. Learn how ice's unique molecular structure and the anomalous expansion of water lead to its lower density and buoyancy. Learn the reason behind the peculiar behavior of ice cubes floating in water, which is due to the decrease in density of water molecules as they turn into ice. Discover the implications of ice floating on. Learn how this phenomenon affects lakes, rivers and fish, and how heavy water ice sinks in. The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. Why does ice float on water? Ice has a hexagonal framework with open spaces that make it less dense than liquid water. Learn why ice floats on water and how the structure of ice differs from liquid water. Water molecules form hydrogen bonds when an oxygen atom (red) in one attracts a hydrogen atom (grey) in another (top). Ice floats because it is less dense than liquid water due to hydrogen bonding.
From www.slideserve.com
PPT L14 Fluids [3] PowerPoint Presentation, free download ID6186946 Why Does Ice Float Molecules Learn the reason behind the peculiar behavior of ice cubes floating in water, which is due to the decrease in density of water molecules as they turn into ice. Learn how this phenomenon affects lakes, rivers and fish, and how heavy water ice sinks in. Water molecules form hydrogen bonds when an oxygen atom (red) in one attracts a hydrogen. Why Does Ice Float Molecules.
From www.slideserve.com
PPT Solids differ Hardness Melting point Flexibility Conductivity PowerPoint Presentation Why Does Ice Float Molecules Discover the implications of ice floating on. Learn how ice's unique molecular structure and the anomalous expansion of water lead to its lower density and buoyancy. Ice floats because it is less dense than liquid water due to hydrogen bonding. Ice has a hexagonal framework with open spaces that make it less dense than liquid water. Learn why ice floats. Why Does Ice Float Molecules.
From www.livescience.com
Why does ice float? Live Science Why Does Ice Float Molecules Water molecules form hydrogen bonds when an oxygen atom (red) in one attracts a hydrogen atom (grey) in another (top). Discover the implications of ice floating on. Why does ice float on water? Ice has a hexagonal framework with open spaces that make it less dense than liquid water. Ice floats because it is less dense than liquid water due. Why Does Ice Float Molecules.
From www.showme.com
Why does ice float? ShowMe Why Does Ice Float Molecules Learn why ice floats on water and how the structure of ice differs from liquid water. Ice has a hexagonal framework with open spaces that make it less dense than liquid water. Why does ice float on water? Learn the reason behind the peculiar behavior of ice cubes floating in water, which is due to the decrease in density of. Why Does Ice Float Molecules.
From www.slideserve.com
PPT The Nature Of Molecules PowerPoint Presentation, free download ID710313 Why Does Ice Float Molecules Discover the implications of ice floating on. Ice floats because it is less dense than liquid water due to hydrogen bonding. Ice has a hexagonal framework with open spaces that make it less dense than liquid water. Learn why ice floats on water and how the structure of ice differs from liquid water. Learn how this phenomenon affects lakes, rivers. Why Does Ice Float Molecules.
From www.slideserve.com
PPT Intermolecular Force PowerPoint Presentation, free download ID234551 Why Does Ice Float Molecules Learn how this phenomenon affects lakes, rivers and fish, and how heavy water ice sinks in. Learn the reason behind the peculiar behavior of ice cubes floating in water, which is due to the decrease in density of water molecules as they turn into ice. Ice has a hexagonal framework with open spaces that make it less dense than liquid. Why Does Ice Float Molecules.
From www.pinterest.com
Why does ice float in water? Zaidan and Charles Morton Chemistry classroom, Physical Why Does Ice Float Molecules Learn how ice's unique molecular structure and the anomalous expansion of water lead to its lower density and buoyancy. Ice floats because it is less dense than liquid water due to hydrogen bonding. Discover the implications of ice floating on. Learn why ice floats on water and how the structure of ice differs from liquid water. Water molecules form hydrogen. Why Does Ice Float Molecules.
From tinyhousegarage.com
Why Does Ice Float On Water Chemistry? Why Does Ice Float Molecules Ice floats because it is less dense than liquid water due to hydrogen bonding. Learn how ice's unique molecular structure and the anomalous expansion of water lead to its lower density and buoyancy. Discover the implications of ice floating on. Why does ice float on water? Learn the reason behind the peculiar behavior of ice cubes floating in water, which. Why Does Ice Float Molecules.
From www.slideserve.com
PPT Chapter 11 Fluid Statics PowerPoint Presentation, free download ID8785740 Why Does Ice Float Molecules Learn how ice's unique molecular structure and the anomalous expansion of water lead to its lower density and buoyancy. Learn how this phenomenon affects lakes, rivers and fish, and how heavy water ice sinks in. Why does ice float on water? Ice floats because it is less dense than liquid water due to hydrogen bonding. Ice has a hexagonal framework. Why Does Ice Float Molecules.
From www.youtube.com
Why ice floats on liquid water? Why volume of water increases on freezing? YouTube Why Does Ice Float Molecules Learn why ice floats on water and how the structure of ice differs from liquid water. Why does ice float on water? Learn how ice's unique molecular structure and the anomalous expansion of water lead to its lower density and buoyancy. Discover the implications of ice floating on. Ice has a hexagonal framework with open spaces that make it less. Why Does Ice Float Molecules.
From www.dreamstime.com
Why Does Ice Float on Water Infographic Diagram Stock Vector Illustration of motion, density Why Does Ice Float Molecules Ice floats because it is less dense than liquid water due to hydrogen bonding. Learn how this phenomenon affects lakes, rivers and fish, and how heavy water ice sinks in. Learn how ice's unique molecular structure and the anomalous expansion of water lead to its lower density and buoyancy. Learn the reason behind the peculiar behavior of ice cubes floating. Why Does Ice Float Molecules.
From www.youtube.com
Why Does Ice Float? YouTube Why Does Ice Float Molecules Why does ice float on water? Ice floats because it is less dense than liquid water due to hydrogen bonding. Ice has a hexagonal framework with open spaces that make it less dense than liquid water. Learn how ice's unique molecular structure and the anomalous expansion of water lead to its lower density and buoyancy. Discover the implications of ice. Why Does Ice Float Molecules.
From www.youtube.com
Why Ice Floats on Water YouTube Why Does Ice Float Molecules Ice floats because it is less dense than liquid water due to hydrogen bonding. Why does ice float on water? Ice has a hexagonal framework with open spaces that make it less dense than liquid water. Water molecules form hydrogen bonds when an oxygen atom (red) in one attracts a hydrogen atom (grey) in another (top). Learn why ice floats. Why Does Ice Float Molecules.
From www.slideserve.com
PPT Water PowerPoint Presentation, free download ID1344926 Why Does Ice Float Molecules The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. Discover the implications of ice floating on. Learn why ice floats on water and how the structure of ice differs from liquid water. Learn how ice's unique molecular structure and the anomalous expansion of water lead to its lower density and buoyancy.. Why Does Ice Float Molecules.
From punchlistzero.com
Specific Heat of Ice In Various Units, vs. Water, Why Does Ice Float Molecules Learn the reason behind the peculiar behavior of ice cubes floating in water, which is due to the decrease in density of water molecules as they turn into ice. Why does ice float on water? The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. Learn why ice floats on water and. Why Does Ice Float Molecules.
From www.slideserve.com
PPT L14 Fluids [3] PowerPoint Presentation, free download ID5631091 Why Does Ice Float Molecules Learn how ice's unique molecular structure and the anomalous expansion of water lead to its lower density and buoyancy. Discover the implications of ice floating on. The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. Why does ice float on water? Water molecules form hydrogen bonds when an oxygen atom (red). Why Does Ice Float Molecules.
From www.reddit.com
ELI5 Why does ice float in water? Isn't ice 'denser' than liquid water? r/explainlikeimfive Why Does Ice Float Molecules Learn the reason behind the peculiar behavior of ice cubes floating in water, which is due to the decrease in density of water molecules as they turn into ice. Discover the implications of ice floating on. Ice has a hexagonal framework with open spaces that make it less dense than liquid water. Learn why ice floats on water and how. Why Does Ice Float Molecules.
From www.slideserve.com
PPT Summary on intermolecular forces PowerPoint Presentation, free download ID432746 Why Does Ice Float Molecules Learn why ice floats on water and how the structure of ice differs from liquid water. Learn how ice's unique molecular structure and the anomalous expansion of water lead to its lower density and buoyancy. Learn the reason behind the peculiar behavior of ice cubes floating in water, which is due to the decrease in density of water molecules as. Why Does Ice Float Molecules.
From slideplayer.com
Water…. ppt download Why Does Ice Float Molecules Learn the reason behind the peculiar behavior of ice cubes floating in water, which is due to the decrease in density of water molecules as they turn into ice. Why does ice float on water? Ice floats because it is less dense than liquid water due to hydrogen bonding. Learn why ice floats on water and how the structure of. Why Does Ice Float Molecules.
From slideplayer.com
Water Unit. ppt download Why Does Ice Float Molecules Learn how ice's unique molecular structure and the anomalous expansion of water lead to its lower density and buoyancy. Ice has a hexagonal framework with open spaces that make it less dense than liquid water. Learn how this phenomenon affects lakes, rivers and fish, and how heavy water ice sinks in. Discover the implications of ice floating on. Why does. Why Does Ice Float Molecules.
From thevigyan.com
Why Does Ice Float in Water? The Vigyan Why Does Ice Float Molecules Ice floats because it is less dense than liquid water due to hydrogen bonding. The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. Discover the implications of ice floating on. Ice has a hexagonal framework with open spaces that make it less dense than liquid water. Learn the reason behind the. Why Does Ice Float Molecules.
From www.youtube.com
Why does ice float in water? Zaidan and Charles Morton YouTube Why Does Ice Float Molecules Learn how ice's unique molecular structure and the anomalous expansion of water lead to its lower density and buoyancy. Discover the implications of ice floating on. Water molecules form hydrogen bonds when an oxygen atom (red) in one attracts a hydrogen atom (grey) in another (top). Learn how this phenomenon affects lakes, rivers and fish, and how heavy water ice. Why Does Ice Float Molecules.
From www.slideserve.com
PPT Ch 1 The Chemistry of Life PowerPoint Presentation, free download ID3501963 Why Does Ice Float Molecules Ice has a hexagonal framework with open spaces that make it less dense than liquid water. Ice floats because it is less dense than liquid water due to hydrogen bonding. Learn how ice's unique molecular structure and the anomalous expansion of water lead to its lower density and buoyancy. The molecules in ice are held further apart by the hydrogen. Why Does Ice Float Molecules.
From www.slideserve.com
PPT Polar Bonds and Molecules PowerPoint Presentation, free download ID3762676 Why Does Ice Float Molecules The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. Learn how this phenomenon affects lakes, rivers and fish, and how heavy water ice sinks in. Ice floats because it is less dense than liquid water due to hydrogen bonding. Learn why ice floats on water and how the structure of ice. Why Does Ice Float Molecules.
From www.slideserve.com
PPT Water, water everywhere! PowerPoint Presentation, free download ID3063710 Why Does Ice Float Molecules Ice floats because it is less dense than liquid water due to hydrogen bonding. Learn how ice's unique molecular structure and the anomalous expansion of water lead to its lower density and buoyancy. Why does ice float on water? Learn how this phenomenon affects lakes, rivers and fish, and how heavy water ice sinks in. Learn why ice floats on. Why Does Ice Float Molecules.
From www.slideserve.com
PPT Marine Biology Lesson 3 PowerPoint Presentation, free download ID1597821 Why Does Ice Float Molecules Learn the reason behind the peculiar behavior of ice cubes floating in water, which is due to the decrease in density of water molecules as they turn into ice. The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. Water molecules form hydrogen bonds when an oxygen atom (red) in one attracts. Why Does Ice Float Molecules.
From higheducations.com
Why Does Ice Float in Liquid Water? Why Does Ice Float Molecules Ice floats because it is less dense than liquid water due to hydrogen bonding. Ice has a hexagonal framework with open spaces that make it less dense than liquid water. Learn how this phenomenon affects lakes, rivers and fish, and how heavy water ice sinks in. Learn why ice floats on water and how the structure of ice differs from. Why Does Ice Float Molecules.
From www.slideserve.com
PPT Summary on intermolecular forces PowerPoint Presentation, free download ID432746 Why Does Ice Float Molecules The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. Ice floats because it is less dense than liquid water due to hydrogen bonding. Water molecules form hydrogen bonds when an oxygen atom (red) in one attracts a hydrogen atom (grey) in another (top). Discover the implications of ice floating on. Learn. Why Does Ice Float Molecules.
From www.numerade.com
SOLVED Why does ice float in liquid water? Ice has tiny " air bubbles in it Hydrogen bonding Why Does Ice Float Molecules Ice has a hexagonal framework with open spaces that make it less dense than liquid water. Learn the reason behind the peculiar behavior of ice cubes floating in water, which is due to the decrease in density of water molecules as they turn into ice. The molecules in ice are held further apart by the hydrogen bonds between the oxygen. Why Does Ice Float Molecules.
From academichelp.net
Why Does Ice Float on Water? The Science Behind It Explained Why Does Ice Float Molecules Learn why ice floats on water and how the structure of ice differs from liquid water. Water molecules form hydrogen bonds when an oxygen atom (red) in one attracts a hydrogen atom (grey) in another (top). Learn how ice's unique molecular structure and the anomalous expansion of water lead to its lower density and buoyancy. Ice floats because it is. Why Does Ice Float Molecules.
From www.slideserve.com
PPT Polar Bonds and Molecules PowerPoint Presentation, free download ID3762676 Why Does Ice Float Molecules Ice has a hexagonal framework with open spaces that make it less dense than liquid water. Water molecules form hydrogen bonds when an oxygen atom (red) in one attracts a hydrogen atom (grey) in another (top). Ice floats because it is less dense than liquid water due to hydrogen bonding. Why does ice float on water? Learn how this phenomenon. Why Does Ice Float Molecules.
From www.slideserve.com
PPT WHY? PowerPoint Presentation, free download ID2511404 Why Does Ice Float Molecules Ice floats because it is less dense than liquid water due to hydrogen bonding. The molecules in ice are held further apart by the hydrogen bonds between the oxygen and hydrogen atoms. Why does ice float on water? Learn the reason behind the peculiar behavior of ice cubes floating in water, which is due to the decrease in density of. Why Does Ice Float Molecules.
From techiescientist.com
Why Does Ice Float on Water? Techiescientist Why Does Ice Float Molecules Water molecules form hydrogen bonds when an oxygen atom (red) in one attracts a hydrogen atom (grey) in another (top). Learn the reason behind the peculiar behavior of ice cubes floating in water, which is due to the decrease in density of water molecules as they turn into ice. Ice has a hexagonal framework with open spaces that make it. Why Does Ice Float Molecules.
From www.youtube.com
Why Does Ice Float?!? Intermolecular Forces Explained Hydrogen Bonding and IonDipole Forces Why Does Ice Float Molecules Learn why ice floats on water and how the structure of ice differs from liquid water. Discover the implications of ice floating on. Water molecules form hydrogen bonds when an oxygen atom (red) in one attracts a hydrogen atom (grey) in another (top). Ice floats because it is less dense than liquid water due to hydrogen bonding. Why does ice. Why Does Ice Float Molecules.
From guernseydonkey.com
Why Does Ice Float? Why Does Ice Float Molecules Ice has a hexagonal framework with open spaces that make it less dense than liquid water. Learn the reason behind the peculiar behavior of ice cubes floating in water, which is due to the decrease in density of water molecules as they turn into ice. Learn why ice floats on water and how the structure of ice differs from liquid. Why Does Ice Float Molecules.