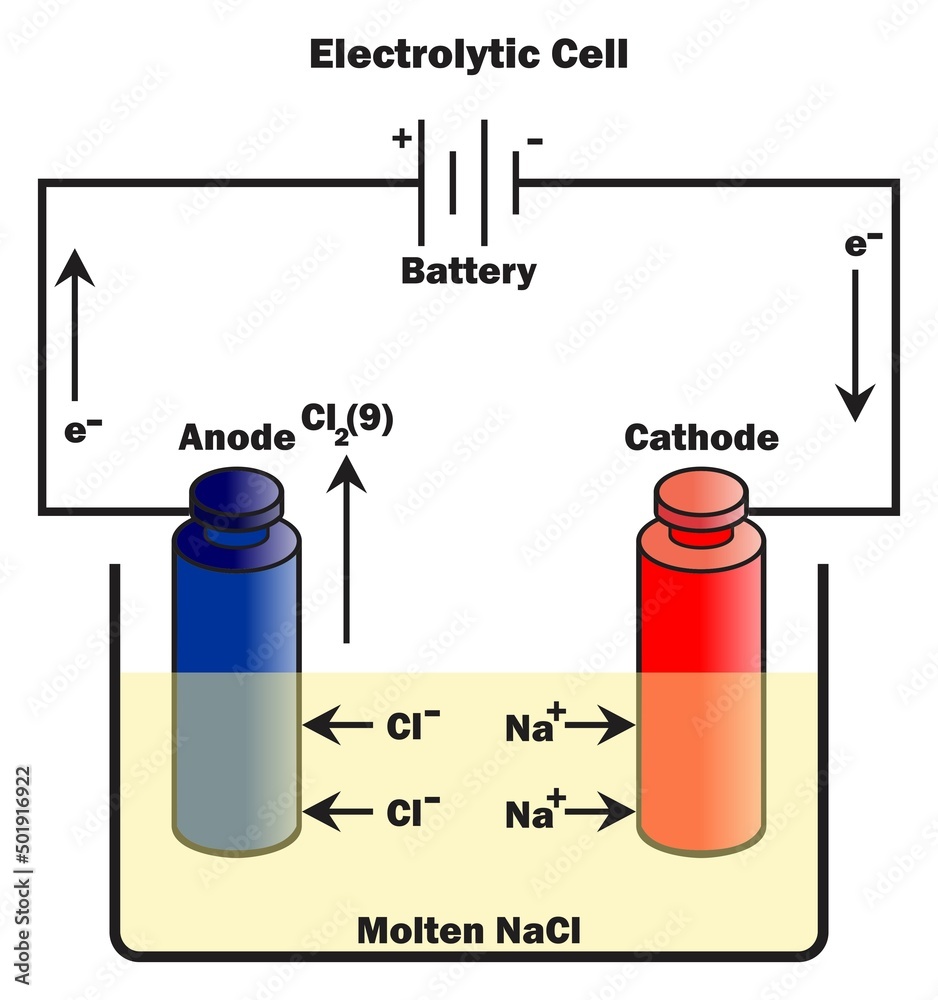
What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell . electrolysis is a fundamental part of chemistry, which involves breaking of electrolyte (the solution) and formation of positive and negative. if molten \(nacl_{(l)}\) is placed into the container and inert electrodes of \(c_{(s)}\) are inserted, attached to the. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. unlike galvanic cells, in an electrolytic cell, the anode is positive as the external battery takes the electrons from it and provides them to the cathode making it. Identify the positive and negative electrodes from diagrams showing the practical set. the second is from the perspective of the external circuit, where the negative electrons flow to the positive terminal, which is the. name the positive and negative electrodes in an electrolytic cell.
from stock.adobe.com
electrolysis is a fundamental part of chemistry, which involves breaking of electrolyte (the solution) and formation of positive and negative. the second is from the perspective of the external circuit, where the negative electrons flow to the positive terminal, which is the. Identify the positive and negative electrodes from diagrams showing the practical set. name the positive and negative electrodes in an electrolytic cell. if molten \(nacl_{(l)}\) is placed into the container and inert electrodes of \(c_{(s)}\) are inserted, attached to the. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. unlike galvanic cells, in an electrolytic cell, the anode is positive as the external battery takes the electrons from it and provides them to the cathode making it.
Electrolytic cell infographic diagram with components including anode
What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell unlike galvanic cells, in an electrolytic cell, the anode is positive as the external battery takes the electrons from it and provides them to the cathode making it. the second is from the perspective of the external circuit, where the negative electrons flow to the positive terminal, which is the. electrolysis is a fundamental part of chemistry, which involves breaking of electrolyte (the solution) and formation of positive and negative. Identify the positive and negative electrodes from diagrams showing the practical set. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. name the positive and negative electrodes in an electrolytic cell. unlike galvanic cells, in an electrolytic cell, the anode is positive as the external battery takes the electrons from it and provides them to the cathode making it. if molten \(nacl_{(l)}\) is placed into the container and inert electrodes of \(c_{(s)}\) are inserted, attached to the.
From electricalacademia.com
Voltaic Cell Working and Construction of Voltaic Cell Electrical What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell Identify the positive and negative electrodes from diagrams showing the practical set. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. if molten \(nacl_{(l)}\) is placed into the container and inert electrodes of \(c_{(s)}\) are inserted, attached to the. unlike galvanic cells, in an electrolytic cell, the anode is positive. What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell.
From ch302.cm.utexas.edu
electrolytic cell What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell Identify the positive and negative electrodes from diagrams showing the practical set. name the positive and negative electrodes in an electrolytic cell. the second is from the perspective of the external circuit, where the negative electrons flow to the positive terminal, which is the. if molten \(nacl_{(l)}\) is placed into the container and inert electrodes of \(c_{(s)}\). What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell.
From bssbibbt.blogspot.com
draw the diagram of electrolytic cell and explain bssbibbt What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell the second is from the perspective of the external circuit, where the negative electrons flow to the positive terminal, which is the. electrolysis is a fundamental part of chemistry, which involves breaking of electrolyte (the solution) and formation of positive and negative. unlike galvanic cells, in an electrolytic cell, the anode is positive as the external battery. What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell.
From www.slideserve.com
PPT CHAPTER 6 ELECTROLYSIS PowerPoint Presentation, free download What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell name the positive and negative electrodes in an electrolytic cell. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. if molten \(nacl_{(l)}\) is placed into the container and inert electrodes of \(c_{(s)}\) are inserted, attached to the. electrolysis is a fundamental part of chemistry, which involves breaking of electrolyte. What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell.
From madisonmeowmercado.blogspot.com
Anode and Cathode in Electrolysis What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell unlike galvanic cells, in an electrolytic cell, the anode is positive as the external battery takes the electrons from it and provides them to the cathode making it. the second is from the perspective of the external circuit, where the negative electrons flow to the positive terminal, which is the. Identify the positive and negative electrodes from diagrams. What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell.
From www.doubtnut.com
Doubt Solutions Maths, Science, CBSE, NCERT, IIT JEE, NEET What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell name the positive and negative electrodes in an electrolytic cell. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. electrolysis is a fundamental part of chemistry, which involves breaking of electrolyte (the solution) and formation of positive and negative. if molten \(nacl_{(l)}\) is placed into the container and inert. What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell.
From stock.adobe.com
Vector scientific illustration of the electrolysis processes. Set of What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell name the positive and negative electrodes in an electrolytic cell. unlike galvanic cells, in an electrolytic cell, the anode is positive as the external battery takes the electrons from it and provides them to the cathode making it. if molten \(nacl_{(l)}\) is placed into the container and inert electrodes of \(c_{(s)}\) are inserted, attached to the. . What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell.
From stock.adobe.com
Electrolytic cell infographic diagram with components including anode What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell if molten \(nacl_{(l)}\) is placed into the container and inert electrodes of \(c_{(s)}\) are inserted, attached to the. name the positive and negative electrodes in an electrolytic cell. unlike galvanic cells, in an electrolytic cell, the anode is positive as the external battery takes the electrons from it and provides them to the cathode making it. Identify. What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell.
From www.pinterest.de
Electrochemistry, featuring electrolysis and fuel cells What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell electrolysis is a fundamental part of chemistry, which involves breaking of electrolyte (the solution) and formation of positive and negative. name the positive and negative electrodes in an electrolytic cell. the second is from the perspective of the external circuit, where the negative electrons flow to the positive terminal, which is the. if molten \(nacl_{(l)}\) is. What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell.
From brilliant.org
Electrolytic Cells and Electrolysis Brilliant Math & Science Wiki What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell the second is from the perspective of the external circuit, where the negative electrons flow to the positive terminal, which is the. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. Identify the positive and negative electrodes from diagrams showing the practical set. unlike galvanic cells, in an electrolytic cell,. What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell.
From schoolbag.info
Electrochemical Cells Electrochemistry Training MCAT General What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell the second is from the perspective of the external circuit, where the negative electrons flow to the positive terminal, which is the. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. name the positive and negative electrodes in an electrolytic cell. Identify the positive and negative electrodes from diagrams showing. What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell.
From hxectqqav.blob.core.windows.net
Electrolytic Electrode Charge at Keith Sullivan blog What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell name the positive and negative electrodes in an electrolytic cell. unlike galvanic cells, in an electrolytic cell, the anode is positive as the external battery takes the electrons from it and provides them to the cathode making it. Identify the positive and negative electrodes from diagrams showing the practical set. in any electrochemical cell (electrolytic or galvanic). What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell.
From library.fiveable.me
Electrolysis Intro to Chemistry Class Notes Fiveable What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell electrolysis is a fundamental part of chemistry, which involves breaking of electrolyte (the solution) and formation of positive and negative. unlike galvanic cells, in an electrolytic cell, the anode is positive as the external battery takes the electrons from it and provides them to the cathode making it. in any electrochemical cell (electrolytic or galvanic) the electrode. What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell.
From byjus.com
An electrolytic cell is used to convert What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell the second is from the perspective of the external circuit, where the negative electrons flow to the positive terminal, which is the. unlike galvanic cells, in an electrolytic cell, the anode is positive as the external battery takes the electrons from it and provides them to the cathode making it. electrolysis is a fundamental part of chemistry,. What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell.
From chemwiki.ucdavis.edu
Electrolytic Cells Chemwiki What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. name the positive and negative electrodes in an electrolytic cell. unlike galvanic cells, in an electrolytic cell, the anode is positive as the external battery takes the electrons from it and provides them to the cathode making it. if molten. What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell.
From classnotes.org.in
Electrolytic Cells Chemistry, Class 12, Electro Chemistry What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell name the positive and negative electrodes in an electrolytic cell. unlike galvanic cells, in an electrolytic cell, the anode is positive as the external battery takes the electrons from it and provides them to the cathode making it. electrolysis is a fundamental part of chemistry, which involves breaking of electrolyte (the solution) and formation of positive and. What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell.
From sciencevision.in
Electrolytes , Electolytic Cell And Electrochemical Cell Science Vision What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell if molten \(nacl_{(l)}\) is placed into the container and inert electrodes of \(c_{(s)}\) are inserted, attached to the. name the positive and negative electrodes in an electrolytic cell. unlike galvanic cells, in an electrolytic cell, the anode is positive as the external battery takes the electrons from it and provides them to the cathode making it. Identify. What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell.
From www.numerade.com
SOLVED 2 Here's an electrolytic cell battery electrodes electrolte What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell if molten \(nacl_{(l)}\) is placed into the container and inert electrodes of \(c_{(s)}\) are inserted, attached to the. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. unlike galvanic cells, in an electrolytic cell, the anode is positive as the external battery takes the electrons from it and provides them. What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell.
From slideplayer.com
Electrochemistry. ppt download What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. if molten \(nacl_{(l)}\) is placed into the container and inert electrodes of \(c_{(s)}\) are inserted, attached to the. Identify the positive and negative electrodes from diagrams showing the practical set. the second is from the perspective of the external circuit, where. What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell.
From courses.lumenlearning.com
Standard Reduction Potentials Chemistry What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell Identify the positive and negative electrodes from diagrams showing the practical set. the second is from the perspective of the external circuit, where the negative electrons flow to the positive terminal, which is the. electrolysis is a fundamental part of chemistry, which involves breaking of electrolyte (the solution) and formation of positive and negative. if molten \(nacl_{(l)}\). What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell.
From www.yaclass.in
Types of Electrochemical Cell and Electrolytic Cell — lesson. Science What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell Identify the positive and negative electrodes from diagrams showing the practical set. name the positive and negative electrodes in an electrolytic cell. unlike galvanic cells, in an electrolytic cell, the anode is positive as the external battery takes the electrons from it and provides them to the cathode making it. the second is from the perspective of. What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell.
From shop.wf-education.com
Understanding Electrolysis What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. electrolysis is a fundamental part of chemistry, which involves breaking of electrolyte (the solution) and formation of positive and negative. if molten \(nacl_{(l)}\) is placed into the container and inert electrodes of \(c_{(s)}\) are inserted, attached to the. Identify the positive. What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell.
From www.theknowledgelibrary.in
Difference Between Cation & Anion The Knowledge Library What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. electrolysis is a fundamental part of chemistry, which involves breaking of electrolyte (the solution) and formation of positive and negative. if molten \(nacl_{(l)}\) is placed into the container and inert electrodes of \(c_{(s)}\) are inserted, attached to the. the second. What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell.
From general.chemistrysteps.com
Electrolysis Chemistry Steps What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell name the positive and negative electrodes in an electrolytic cell. the second is from the perspective of the external circuit, where the negative electrons flow to the positive terminal, which is the. Identify the positive and negative electrodes from diagrams showing the practical set. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs. What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell.
From www.nagwa.com
Question Video Recalling the Name of the Positive Electrode in an What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. Identify the positive and negative electrodes from diagrams showing the practical set. unlike galvanic cells, in an electrolytic cell, the anode is positive as the external battery takes the electrons from it and provides them to the cathode making it. the. What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell.
From www.youtube.com
Cathode and Anode Quick differences and comparisons YouTube What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell electrolysis is a fundamental part of chemistry, which involves breaking of electrolyte (the solution) and formation of positive and negative. name the positive and negative electrodes in an electrolytic cell. Identify the positive and negative electrodes from diagrams showing the practical set. the second is from the perspective of the external circuit, where the negative electrons flow. What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell.
From giodwrxvk.blob.core.windows.net
Electrodes In Electrolysis at Lindsay Macy blog What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell Identify the positive and negative electrodes from diagrams showing the practical set. electrolysis is a fundamental part of chemistry, which involves breaking of electrolyte (the solution) and formation of positive and negative. unlike galvanic cells, in an electrolytic cell, the anode is positive as the external battery takes the electrons from it and provides them to the cathode. What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell electrolysis is a fundamental part of chemistry, which involves breaking of electrolyte (the solution) and formation of positive and negative. name the positive and negative electrodes in an electrolytic cell. if molten \(nacl_{(l)}\) is placed into the container and inert electrodes of \(c_{(s)}\) are inserted, attached to the. Identify the positive and negative electrodes from diagrams showing. What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell.
From byjus.com
In electrolysis what are the positive and negative electrodes known as?? What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell if molten \(nacl_{(l)}\) is placed into the container and inert electrodes of \(c_{(s)}\) are inserted, attached to the. Identify the positive and negative electrodes from diagrams showing the practical set. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. electrolysis is a fundamental part of chemistry, which involves breaking of. What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell.
From chem2u.blogspot.com
chem2U Elelctrolytic Cell and Chemical Cells What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell Identify the positive and negative electrodes from diagrams showing the practical set. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. if molten \(nacl_{(l)}\) is placed into the container and inert electrodes of \(c_{(s)}\) are inserted, attached to the. name the positive and negative electrodes in an electrolytic cell. . What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell.
From slideplayer.com
Electrochemistry Chapter ppt download What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell name the positive and negative electrodes in an electrolytic cell. the second is from the perspective of the external circuit, where the negative electrons flow to the positive terminal, which is the. electrolysis is a fundamental part of chemistry, which involves breaking of electrolyte (the solution) and formation of positive and negative. unlike galvanic cells, in. What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell.
From www.teachoo.com
Electrolytic Cell Definition, Components, Examples Teachoo What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell unlike galvanic cells, in an electrolytic cell, the anode is positive as the external battery takes the electrons from it and provides them to the cathode making it. if molten \(nacl_{(l)}\) is placed into the container and inert electrodes of \(c_{(s)}\) are inserted, attached to the. in any electrochemical cell (electrolytic or galvanic) the electrode at which. What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell.
From question.pandai.org
Electrolytic Cell What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell the second is from the perspective of the external circuit, where the negative electrons flow to the positive terminal, which is the. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. name the positive and negative electrodes in an electrolytic cell. unlike galvanic cells, in an electrolytic cell, the. What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell.
From www.sliderbase.com
Electrochemical Terminology What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell electrolysis is a fundamental part of chemistry, which involves breaking of electrolyte (the solution) and formation of positive and negative. unlike galvanic cells, in an electrolytic cell, the anode is positive as the external battery takes the electrons from it and provides them to the cathode making it. if molten \(nacl_{(l)}\) is placed into the container and. What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell.
From exoxpbgzu.blob.core.windows.net
Define Electrodes In Chemistry Terms at Monte Cordell blog What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell Identify the positive and negative electrodes from diagrams showing the practical set. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. unlike galvanic cells, in an electrolytic cell, the anode is positive as the external battery takes the electrons from it and provides them to the cathode making it. if. What Are The Names Of The Positive And Negative Electrodes Of An Electrolytic Cell.