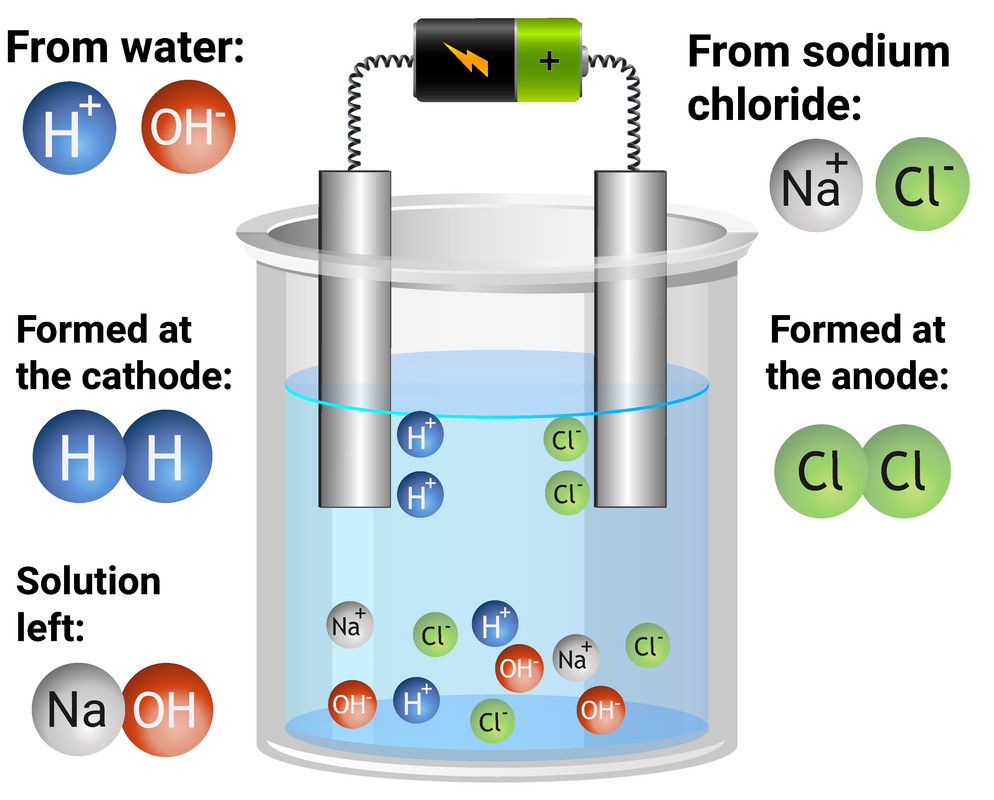
Zinc Carbonate Electrolysis . revision notes on half equations in electrolysis for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. electrolysis involves using electricity to break down electrolytes to form elements. reactive metals are extracted from their ores using electrolysis. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. Ionic compounds conduct electricity when molten or in. metals more reactive than carbon, such as aluminium, are extracted by electrolysis, while metals less reactive than carbon,. The products of electrolysis can be predicted for a given. zinc carbonate nanoparticles with different sizes were electrodeposited by electrolysis of a zinc plate as anode in the. using electricity to force a nonspontaneous process to occur is electrolysis.
from fyooalhxu.blob.core.windows.net
metals more reactive than carbon, such as aluminium, are extracted by electrolysis, while metals less reactive than carbon,. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. reactive metals are extracted from their ores using electrolysis. Ionic compounds conduct electricity when molten or in. revision notes on half equations in electrolysis for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. zinc carbonate nanoparticles with different sizes were electrodeposited by electrolysis of a zinc plate as anode in the. The products of electrolysis can be predicted for a given. using electricity to force a nonspontaneous process to occur is electrolysis. electrolysis involves using electricity to break down electrolytes to form elements.
How Do You Make Electrolyte Solution at Deborah Pellerin blog
Zinc Carbonate Electrolysis revision notes on half equations in electrolysis for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. Ionic compounds conduct electricity when molten or in. revision notes on half equations in electrolysis for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. using electricity to force a nonspontaneous process to occur is electrolysis. zinc carbonate nanoparticles with different sizes were electrodeposited by electrolysis of a zinc plate as anode in the. metals more reactive than carbon, such as aluminium, are extracted by electrolysis, while metals less reactive than carbon,. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. electrolysis involves using electricity to break down electrolytes to form elements. The products of electrolysis can be predicted for a given. reactive metals are extracted from their ores using electrolysis.
From www.researchgate.net
Schematic illustration of a) the slow electrolysis of the zinc powder Zinc Carbonate Electrolysis reactive metals are extracted from their ores using electrolysis. electrolysis involves using electricity to break down electrolytes to form elements. Ionic compounds conduct electricity when molten or in. using electricity to force a nonspontaneous process to occur is electrolysis. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force. Zinc Carbonate Electrolysis.
From www.alamy.com
Modern version of the Leclanche cell. This heavyduty zinccarbon Stock Zinc Carbonate Electrolysis electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. zinc carbonate nanoparticles with different sizes were electrodeposited by electrolysis of a zinc plate as anode in the. reactive metals are extracted from their ores using electrolysis. revision notes on half equations in. Zinc Carbonate Electrolysis.
From www.youtube.com
Type of Reaction for ZnCO3 = ZnO + CO2 YouTube Zinc Carbonate Electrolysis electrolysis involves using electricity to break down electrolytes to form elements. revision notes on half equations in electrolysis for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. Ionic compounds conduct electricity when molten or in. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to. Zinc Carbonate Electrolysis.
From www.coursehero.com
Electrolysis Chemistry for Majors Atoms First Course Hero Zinc Carbonate Electrolysis electrolysis involves using electricity to break down electrolytes to form elements. reactive metals are extracted from their ores using electrolysis. The products of electrolysis can be predicted for a given. revision notes on half equations in electrolysis for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. zinc carbonate nanoparticles with. Zinc Carbonate Electrolysis.
From www.911metallurgist.com
Zinc Electrolysis Zinc Carbonate Electrolysis reactive metals are extracted from their ores using electrolysis. Ionic compounds conduct electricity when molten or in. using electricity to force a nonspontaneous process to occur is electrolysis. revision notes on half equations in electrolysis for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. electrolysis involves using electricity to break. Zinc Carbonate Electrolysis.
From chempedia.info
Zinc carbonate, water reactions Big Chemical Encyclopedia Zinc Carbonate Electrolysis reactive metals are extracted from their ores using electrolysis. electrolysis involves using electricity to break down electrolytes to form elements. revision notes on half equations in electrolysis for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. Ionic compounds conduct electricity when molten or in. zinc carbonate nanoparticles with different sizes. Zinc Carbonate Electrolysis.
From 2012books.lardbucket.org
Electrochemistry Zinc Carbonate Electrolysis zinc carbonate nanoparticles with different sizes were electrodeposited by electrolysis of a zinc plate as anode in the. revision notes on half equations in electrolysis for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. electrolysis involves using electricity to break down electrolytes to form elements. reactive metals are extracted from. Zinc Carbonate Electrolysis.
From eplating.co.uk
Zinc Plating Electrolyte ePlating Zinc Carbonate Electrolysis metals more reactive than carbon, such as aluminium, are extracted by electrolysis, while metals less reactive than carbon,. revision notes on half equations in electrolysis for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. using electricity to force a nonspontaneous process to occur is electrolysis. electrolysis can occur in electrolytic. Zinc Carbonate Electrolysis.
From saylordotorg.github.io
Electrolysis Zinc Carbonate Electrolysis using electricity to force a nonspontaneous process to occur is electrolysis. metals more reactive than carbon, such as aluminium, are extracted by electrolysis, while metals less reactive than carbon,. The products of electrolysis can be predicted for a given. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the. Zinc Carbonate Electrolysis.
From chemisting.com
Zinc Bromine Batteries First results ever using Propylene Carbonate Zinc Carbonate Electrolysis electrolysis involves using electricity to break down electrolytes to form elements. metals more reactive than carbon, such as aluminium, are extracted by electrolysis, while metals less reactive than carbon,. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. The products of electrolysis can. Zinc Carbonate Electrolysis.
From www.researchgate.net
Raman spectra of the cemented product formed using zinc dust in 0.5 M Zinc Carbonate Electrolysis zinc carbonate nanoparticles with different sizes were electrodeposited by electrolysis of a zinc plate as anode in the. revision notes on half equations in electrolysis for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. electrolysis involves using electricity to break down electrolytes to form elements. The products of electrolysis can be. Zinc Carbonate Electrolysis.
From pubs.sciepub.com
Figure 14. CuZn battery inside the beaker with the stirred electrolyte Zinc Carbonate Electrolysis electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. The products of electrolysis can be predicted for a given. electrolysis involves using electricity to break down electrolytes to form elements. reactive metals are extracted from their ores using electrolysis. zinc carbonate nanoparticles. Zinc Carbonate Electrolysis.
From chem.libretexts.org
20.4 Cell Potential Under Standard Conditions Chemistry LibreTexts Zinc Carbonate Electrolysis electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. The products of electrolysis can be predicted for a given. revision notes on half equations in electrolysis for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. electrolysis involves. Zinc Carbonate Electrolysis.
From www.teachoo.com
Reactions of Acids and Bases Full list (with Examples) Teachoo Zinc Carbonate Electrolysis metals more reactive than carbon, such as aluminium, are extracted by electrolysis, while metals less reactive than carbon,. electrolysis involves using electricity to break down electrolytes to form elements. The products of electrolysis can be predicted for a given. reactive metals are extracted from their ores using electrolysis. electrolysis can occur in electrolytic cells by introducing. Zinc Carbonate Electrolysis.
From fyooalhxu.blob.core.windows.net
How Do You Make Electrolyte Solution at Deborah Pellerin blog Zinc Carbonate Electrolysis zinc carbonate nanoparticles with different sizes were electrodeposited by electrolysis of a zinc plate as anode in the. revision notes on half equations in electrolysis for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. reactive metals are extracted from their ores using electrolysis. using electricity to force a nonspontaneous process. Zinc Carbonate Electrolysis.
From edu.rsc.org
Electrolysis of molten zinc chloride Resource RSC Education Zinc Carbonate Electrolysis revision notes on half equations in electrolysis for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. zinc carbonate nanoparticles with different sizes were electrodeposited by electrolysis of a zinc plate as anode in the. reactive metals are extracted from their ores using electrolysis. The products of electrolysis can be predicted for. Zinc Carbonate Electrolysis.
From www.911metallurgist.com
How to Recover Zinc from Zinc Chloride by Electrolysis Zinc Carbonate Electrolysis using electricity to force a nonspontaneous process to occur is electrolysis. The products of electrolysis can be predicted for a given. reactive metals are extracted from their ores using electrolysis. electrolysis involves using electricity to break down electrolytes to form elements. zinc carbonate nanoparticles with different sizes were electrodeposited by electrolysis of a zinc plate as. Zinc Carbonate Electrolysis.
From iolitec.de
Zincair batteries IoLiTec Zinc Carbonate Electrolysis Ionic compounds conduct electricity when molten or in. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. zinc carbonate nanoparticles with different sizes were electrodeposited by electrolysis of a zinc plate as anode in the. reactive metals are extracted from their ores using. Zinc Carbonate Electrolysis.
From www.youtube.com
Calculating Ksp of Zinc Carbonate using electrode potentials YouTube Zinc Carbonate Electrolysis reactive metals are extracted from their ores using electrolysis. using electricity to force a nonspontaneous process to occur is electrolysis. metals more reactive than carbon, such as aluminium, are extracted by electrolysis, while metals less reactive than carbon,. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the. Zinc Carbonate Electrolysis.
From www.911metallurgist.com
How to Recover Zinc from Zinc Chloride by Electrolysis Zinc Carbonate Electrolysis revision notes on half equations in electrolysis for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. electrolysis involves using electricity to break down electrolytes to form elements. metals more reactive than carbon, such as aluminium, are extracted by electrolysis, while metals less reactive than carbon,. zinc carbonate nanoparticles with different. Zinc Carbonate Electrolysis.
From www.researchgate.net
Electrolysis on Zinc cathodes at 45 °C, 10 bar CO2 applied current Zinc Carbonate Electrolysis Ionic compounds conduct electricity when molten or in. electrolysis involves using electricity to break down electrolytes to form elements. revision notes on half equations in electrolysis for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. metals more reactive than carbon, such as aluminium, are extracted by electrolysis, while metals less reactive. Zinc Carbonate Electrolysis.
From www.911metallurgist.com
How to Recover Zinc from Zinc Chloride by Electrolysis Zinc Carbonate Electrolysis reactive metals are extracted from their ores using electrolysis. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. using electricity to force a nonspontaneous process to occur is electrolysis. metals more reactive than carbon, such as aluminium, are extracted by electrolysis, while. Zinc Carbonate Electrolysis.
From pubs.rsc.org
Direct carbonate electrolysis into pure syngas EES Catalysis (RSC Zinc Carbonate Electrolysis reactive metals are extracted from their ores using electrolysis. revision notes on half equations in electrolysis for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. zinc carbonate. Zinc Carbonate Electrolysis.
From www.researchgate.net
Strategy to realize (bi)carbonate electrolysis. (a) Schematic Zinc Carbonate Electrolysis using electricity to force a nonspontaneous process to occur is electrolysis. Ionic compounds conduct electricity when molten or in. reactive metals are extracted from their ores using electrolysis. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. metals more reactive than carbon,. Zinc Carbonate Electrolysis.
From www.researchgate.net
Performance of the direct carbonate electrolysis cell. (a) Full cell Zinc Carbonate Electrolysis The products of electrolysis can be predicted for a given. revision notes on half equations in electrolysis for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. electrolysis involves using electricity to break down electrolytes to form elements. Ionic compounds conduct electricity when molten or in. reactive metals are extracted from their. Zinc Carbonate Electrolysis.
From pubs.rsc.org
Electrochemical conversion of CO 2 into valueadded carbon with Zinc Carbonate Electrolysis using electricity to force a nonspontaneous process to occur is electrolysis. reactive metals are extracted from their ores using electrolysis. Ionic compounds conduct electricity when molten or in. electrolysis involves using electricity to break down electrolytes to form elements. The products of electrolysis can be predicted for a given. zinc carbonate nanoparticles with different sizes were. Zinc Carbonate Electrolysis.
From fyopepcgh.blob.core.windows.net
Do Bases React With Zinc at Ramon Thomas blog Zinc Carbonate Electrolysis revision notes on half equations in electrolysis for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. using electricity to force a nonspontaneous process to occur is electrolysis. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. . Zinc Carbonate Electrolysis.
From www.youtube.com
UK GCSE Chemistry Topic 1 Zinc Carbonate YouTube Zinc Carbonate Electrolysis metals more reactive than carbon, such as aluminium, are extracted by electrolysis, while metals less reactive than carbon,. reactive metals are extracted from their ores using electrolysis. The products of electrolysis can be predicted for a given. revision notes on half equations in electrolysis for the aqa gcse chemistry syllabus, written by the chemistry experts at save. Zinc Carbonate Electrolysis.
From www.youtube.com
How to write chemical formula of zinc carbonateMolecular formula of Zinc Carbonate Electrolysis reactive metals are extracted from their ores using electrolysis. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. zinc carbonate nanoparticles with different sizes were electrodeposited by electrolysis of a zinc plate as anode in the. metals more reactive than carbon, such. Zinc Carbonate Electrolysis.
From www.researchgate.net
(a) Carbon loss mechanisms in a CO2 electrolysis cell with gasfed CO2 Zinc Carbonate Electrolysis using electricity to force a nonspontaneous process to occur is electrolysis. electrolysis involves using electricity to break down electrolytes to form elements. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. Ionic compounds conduct electricity when molten or in. reactive metals are. Zinc Carbonate Electrolysis.
From www.researchgate.net
STEP carbon capture in which three molten carbonate electrolysis in Zinc Carbonate Electrolysis reactive metals are extracted from their ores using electrolysis. Ionic compounds conduct electricity when molten or in. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. revision notes on half equations in electrolysis for the aqa gcse chemistry syllabus, written by the chemistry. Zinc Carbonate Electrolysis.
From testbook.com
Zinc Carbonate Learn its Formula, Structure, Properties & Uses Zinc Carbonate Electrolysis revision notes on half equations in electrolysis for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. using electricity to force a nonspontaneous process to occur is electrolysis. metals more reactive than carbon, such as aluminium, are extracted by electrolysis, while metals less reactive than carbon,. The products of electrolysis can be. Zinc Carbonate Electrolysis.
From www.nagwa.com
Question Video Identifying the Correct Chemical Equation for the Zinc Carbonate Electrolysis Ionic compounds conduct electricity when molten or in. metals more reactive than carbon, such as aluminium, are extracted by electrolysis, while metals less reactive than carbon,. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. using electricity to force a nonspontaneous process to. Zinc Carbonate Electrolysis.
From www.mdpi.com
Applied Sciences Free FullText Carbonation Resistance of Mortar Zinc Carbonate Electrolysis Ionic compounds conduct electricity when molten or in. using electricity to force a nonspontaneous process to occur is electrolysis. The products of electrolysis can be predicted for a given. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. electrolysis involves using electricity to. Zinc Carbonate Electrolysis.
From www.slideshare.net
Chapter 6 electrochemistry Zinc Carbonate Electrolysis revision notes on half equations in electrolysis for the aqa gcse chemistry syllabus, written by the chemistry experts at save my exams. zinc carbonate nanoparticles with different sizes were electrodeposited by electrolysis of a zinc plate as anode in the. using electricity to force a nonspontaneous process to occur is electrolysis. electrolysis involves using electricity to. Zinc Carbonate Electrolysis.