Dilution Factor Solution Chemistry . Concentration is the process of. dilution is the process of adding a solvent to a solution to reduce the concentration of the solute. Use the dilution equation or ideal dilution equation. You may come across something like, prepare a 1:50 dilution of the solution. in chemistry and biology, the dilution ratio and dilution factor are two related (but slightly different) expressions of the. You divide the final volume by the. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Often, a worker will need to change the concentration of a solution by changing the amount. Many solutions contain one component, called the solvent, in which. Solutions can be prepared by diluting a more concentrated solution rather than starting. Explain how concentrations can be changed in the lab. using dilution factors. the dilution factor represents the ratio of the final volume of the diluted solution to the initial volume of the concentrated. How does dilution affect the reaction rate? free online dilution calculator.
from www.youtube.com
solutions are homogeneous mixtures. definition of dilution factor. dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more. Explain how concentrations can be changed in the lab. Understand how stock solutions are used. Solutions can be prepared by diluting a more concentrated solution rather than starting. You may come across something like, prepare a 1:50 dilution of the solution. using dilution factors. Concentration is the process of. the solution dilution calculator will calculate for you how to dilute a stock solution of known concentration to obtain.
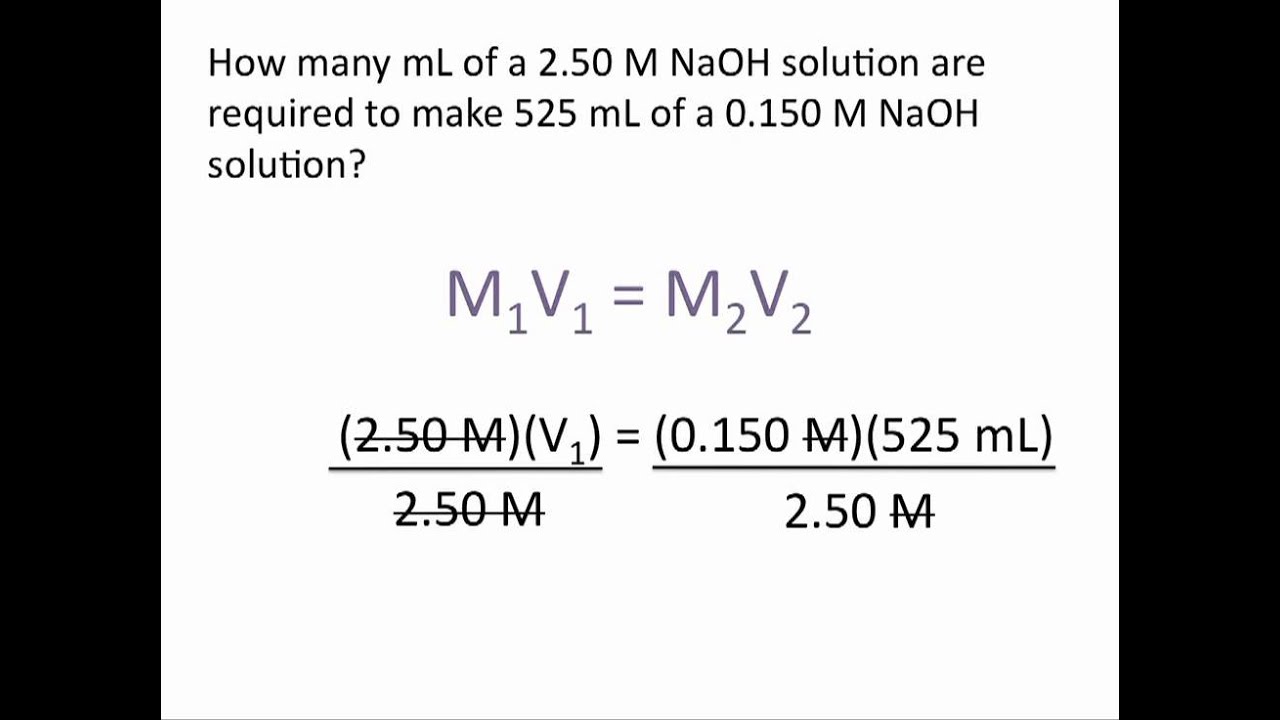
Dilution Problems Chemistry Tutorial YouTube
Dilution Factor Solution Chemistry dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more. Explain how concentrations can be changed in the lab. learn how to dilute and concentrate solutions. free online dilution calculator. Concentration is the process of. Compute the initial or final concentration. Use the dilution equation or ideal dilution equation. under the highest dse condition, high separation factors of se were also obtained, therefore, these results suggest. dilution factor is the ratio of the solute to solvent or simply the ratio of the volume of the initial solution to the volume of the. dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more. in chemistry and biology, the dilution ratio and dilution factor are two related (but slightly different) expressions of the. This way of expressing a dilution as a ratio of the parts of solute to the total number of parts. How does dilution affect the reaction rate? learn how to dilute solutions. state whether the concentration of a solution is directly or indirectly proportional to its volume. chemistry solutions dilution calculations.
From promolasopa510.weebly.com
Calculate dilution factor promolasopa Dilution Factor Solution Chemistry state whether the concentration of a solution is directly or indirectly proportional to its volume. dilution is a process used to lower the concentration of the original solution by adding more solvent. in chemistry and biology, the dilution ratio and dilution factor are two related (but slightly different) expressions of the. the solution dilution calculator will. Dilution Factor Solution Chemistry.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilution Factor Solution Chemistry a common method of making a solution of a given concentration involves taking a more concentration solution and adding water. the main difference between dilution and dilution factor is that dilution is the process of reducing the concentration. Concentration is the process of. state whether the concentration of a solution is directly or indirectly proportional to its. Dilution Factor Solution Chemistry.
From www.hemocytometer.org
Using the dilution factor to calculate dilutions • Hemocytometer Dilution Factor Solution Chemistry dilution is the process of lowering the concentration of a solute in a solution by adding more solvents to the solution, such. Understand how stock solutions are used. the solution dilution calculator will calculate for you how to dilute a stock solution of known concentration to obtain. state whether the concentration of a solution is directly or. Dilution Factor Solution Chemistry.
From dxocmttnm.blob.core.windows.net
Dilution Equation Formulas at Kathleen Milford blog Dilution Factor Solution Chemistry dilution is the process of lowering the concentration of a solute in a solution by adding more solvents to the solution, such. dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. chemistry solutions dilution calculations. Often, a worker will need to change the concentration of a solution by changing. Dilution Factor Solution Chemistry.
From chem370.github.io
Lab 1 Prelab CHEM 370 Dilution Factor Solution Chemistry Understand how stock solutions are used. chemistry solutions dilution calculations. dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more. using dilution factors. Compute the initial or final concentration. How does dilution affect the reaction rate? solutions are homogeneous mixtures. dilution factor is the ratio. Dilution Factor Solution Chemistry.
From sciencestruck.com
Here's How to Calculate Dilution Factor from Given Concentration Dilution Factor Solution Chemistry Explain how concentrations can be changed in the lab. the dilution factor represents the ratio of the final volume of the diluted solution to the initial volume of the concentrated. dilution factor is the ratio of the solute to solvent or simply the ratio of the volume of the initial solution to the volume of the. chemistry. Dilution Factor Solution Chemistry.
From microbenotes.com
Serial Dilution Formula, Calculator, Method, Uses, Examples Dilution Factor Solution Chemistry a common method of making a solution of a given concentration involves taking a more concentration solution and adding water. learn how to dilute and concentrate solutions. Solutions can be prepared by diluting a more concentrated solution rather than starting. Often, a worker will need to change the concentration of a solution by changing the amount. a. Dilution Factor Solution Chemistry.
From hive.blog
Dilution factor — Hive Dilution Factor Solution Chemistry using dilution factors. the solution dilution calculator will calculate for you how to dilute a stock solution of known concentration to obtain. Many solutions contain one component, called the solvent, in which. This way of expressing a dilution as a ratio of the parts of solute to the total number of parts. solutions are homogeneous mixtures. Often,. Dilution Factor Solution Chemistry.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube Dilution Factor Solution Chemistry Solutions can be prepared by diluting a more concentrated solution rather than starting. dilution is a process used to lower the concentration of the original solution by adding more solvent. free online dilution calculator. Understand how stock solutions are used. the dilution factor (or dilution ratio) is the notation used to express how much of the original. Dilution Factor Solution Chemistry.
From www.youtube.com
A Level Chemistry Dilution Calculations Worked Example YouTube Dilution Factor Solution Chemistry in chemistry and biology, the dilution ratio and dilution factor are two related (but slightly different) expressions of the. Concentration is the process of. dilution is the process of lowering the concentration of a solute in a solution by adding more solvents to the solution, such. a dilute solution is one in which there is a relatively. Dilution Factor Solution Chemistry.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilution Factor Solution Chemistry state whether the concentration of a solution is directly or indirectly proportional to its volume. dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. chemistry solutions dilution calculations. You may come across something like, prepare a 1:50 dilution of the solution. the main difference between dilution and dilution. Dilution Factor Solution Chemistry.
From www.youtube.com
How to Calculate Dilution Factor YouTube Dilution Factor Solution Chemistry Explain how concentrations can be changed in the lab. Many solutions contain one component, called the solvent, in which. dilution is the process of lowering the concentration of a solute in a solution by adding more solvents to the solution, such. Compute the initial or final concentration. dilution factor is the ratio of the solute to solvent or. Dilution Factor Solution Chemistry.
From microbeonline.com
Serial Dilution Method for Estimating Viable Count of Bacteria Dilution Factor Solution Chemistry You divide the final volume by the. state whether the concentration of a solution is directly or indirectly proportional to its volume. chemistry solutions dilution calculations. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. dilution is the process of decreasing the concentration of a solute. Dilution Factor Solution Chemistry.
From dxogqmfyq.blob.core.windows.net
Does Dilution Increase Concentration at Shawn Burgess blog Dilution Factor Solution Chemistry state whether the concentration of a solution is directly or indirectly proportional to its volume. chemistry solutions dilution calculations. under the highest dse condition, high separation factors of se were also obtained, therefore, these results suggest. chemistry solutions dilution calculations. Explain how concentrations can be changed in the lab. the solution dilution calculator will calculate. Dilution Factor Solution Chemistry.
From www.youtube.com
Dilution Calculation Practice YouTube Dilution Factor Solution Chemistry Concentration is the process of. dilution is a process used to lower the concentration of the original solution by adding more solvent. state whether the concentration of a solution is directly or indirectly proportional to its volume. Use the dilution equation or ideal dilution equation. Compute the initial or final concentration. definition of dilution factor. chemistry. Dilution Factor Solution Chemistry.
From twinklsecondary.blog
Products of a Dilution Series A Level Biology Revision Dilution Factor Solution Chemistry solutions are homogeneous mixtures. learn how to dilute solutions. Explain how concentrations can be changed in the lab. Use the dilution equation or ideal dilution equation. the dilution factor represents the ratio of the final volume of the diluted solution to the initial volume of the concentrated. under the highest dse condition, high separation factors of. Dilution Factor Solution Chemistry.
From www.youtube.com
How to Calculate CFUSerial Dilution Microbiology Technique knowledge Dilution Factor Solution Chemistry in chemistry and biology, the dilution ratio and dilution factor are two related (but slightly different) expressions of the. a dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. state whether the concentration of a solution is directly or indirectly proportional to its volume. using dilution factors.. Dilution Factor Solution Chemistry.
From www.youtube.com
CHEMISTRY 101 Solution Dilutions YouTube Dilution Factor Solution Chemistry This way of expressing a dilution as a ratio of the parts of solute to the total number of parts. the dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total. under the highest dse condition, high separation factors of se were also obtained, therefore,. Dilution Factor Solution Chemistry.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory Dilution Factor Solution Chemistry dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more. the solution dilution calculator will calculate for you how to dilute a stock solution of known concentration to obtain. the main difference between dilution and dilution factor is that dilution is the process of reducing the concentration.. Dilution Factor Solution Chemistry.
From exofyyqul.blob.core.windows.net
Dilutions Made Easy at Kimberly Surles blog Dilution Factor Solution Chemistry chemistry solutions dilution calculations. using dilution factors. in chemistry and biology, the dilution ratio and dilution factor are two related (but slightly different) expressions of the. Often, a worker will need to change the concentration of a solution by changing the amount. solutions are homogeneous mixtures. a common method of making a solution of a. Dilution Factor Solution Chemistry.
From sciencequery.com
What is serial dilution method? And how to calculate? Science Query Dilution Factor Solution Chemistry dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. You may come across something like, prepare a 1:50 dilution of the solution. using dilution factors. Explain how concentrations can be changed in the lab. dilution is the process of decreasing the concentration of a solute in a solution, usually. Dilution Factor Solution Chemistry.
From materialschoolpinder.z21.web.core.windows.net
How To Do Dilutions In Chemistry Dilution Factor Solution Chemistry free online dilution calculator. the solution dilution calculator will calculate for you how to dilute a stock solution of known concentration to obtain. learn how to dilute solutions. using dilution factors. dilution factor is the ratio of the solute to solvent or simply the ratio of the volume of the initial solution to the volume. Dilution Factor Solution Chemistry.
From www.majordifferences.com
Difference between Dilution and Dilution Factor in Microbiology Dilution Factor Solution Chemistry learn how to dilute solutions. Solutions can be prepared by diluting a more concentrated solution rather than starting. under the highest dse condition, high separation factors of se were also obtained, therefore, these results suggest. using dilution factors. the dilution factor (or dilution ratio) is the notation used to express how much of the original stock. Dilution Factor Solution Chemistry.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory Dilution Factor Solution Chemistry dilution is the process of adding a solvent to a solution to reduce the concentration of the solute. dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. dilution factor is the ratio of the solute to solvent or simply the ratio of the volume of the initial solution to. Dilution Factor Solution Chemistry.
From www.youtube.com
Chemistry 11 Dilution Calculations Solving for Initial Concentration Dilution Factor Solution Chemistry You divide the final volume by the. state whether the concentration of a solution is directly or indirectly proportional to its volume. dilution is a process used to lower the concentration of the original solution by adding more solvent. under the highest dse condition, high separation factors of se were also obtained, therefore, these results suggest. . Dilution Factor Solution Chemistry.
From www.youtube.com
TRU Chemistry Labs How To do Dilution Calculations YouTube Dilution Factor Solution Chemistry Understand how stock solutions are used. the dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total. chemistry solutions dilution calculations. state whether the concentration of a solution is directly or indirectly proportional to its volume. dilution is the process of decreasing the. Dilution Factor Solution Chemistry.
From dxorvxlft.blob.core.windows.net
Serial Dilutions Lab at Joseph Brown blog Dilution Factor Solution Chemistry Compute the initial or final concentration. under the highest dse condition, high separation factors of se were also obtained, therefore, these results suggest. the dilution factor represents the ratio of the final volume of the diluted solution to the initial volume of the concentrated. using dilution factors. dilution is the process whereby the concentration of a. Dilution Factor Solution Chemistry.
From kayliefernandez.blogspot.com
PreAP Chemistry Dilutions Dilution Factor Solution Chemistry Understand how stock solutions are used. Compute the initial or final concentration. using dilution factors. free online dilution calculator. You divide the final volume by the. chemistry solutions dilution calculations. the solution dilution calculator will calculate for you how to dilute a stock solution of known concentration to obtain. solutions are homogeneous mixtures. in. Dilution Factor Solution Chemistry.
From mmerevise.co.uk
Concentrations and Dilutions MME Dilution Factor Solution Chemistry the solution dilution calculator will calculate for you how to dilute a stock solution of known concentration to obtain. Compute the initial or final concentration. solutions are homogeneous mixtures. in chemistry and biology, the dilution ratio and dilution factor are two related (but slightly different) expressions of the. dilution factor is the ratio of the solute. Dilution Factor Solution Chemistry.
From socratic.org
How can I calculate the dilution factor using concentration? Socratic Dilution Factor Solution Chemistry under the highest dse condition, high separation factors of se were also obtained, therefore, these results suggest. You may come across something like, prepare a 1:50 dilution of the solution. This way of expressing a dilution as a ratio of the parts of solute to the total number of parts. Understand how stock solutions are used. using dilution. Dilution Factor Solution Chemistry.
From www.youtube.com
Dilution and Dilution Factor in Microbiology How to Calculate Dilution Factor Solution Chemistry Often, a worker will need to change the concentration of a solution by changing the amount. Many solutions contain one component, called the solvent, in which. in chemistry and biology, the dilution ratio and dilution factor are two related (but slightly different) expressions of the. Use the dilution equation or ideal dilution equation. You may come across something like,. Dilution Factor Solution Chemistry.
From www.youtube.com
Dilution Chart.Helpful video. Understand how to prepare dilutions in Dilution Factor Solution Chemistry You divide the final volume by the. definition of dilution factor. the dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total. This way of expressing a dilution as a ratio of the parts of solute to the total number of parts. dilution is. Dilution Factor Solution Chemistry.
From studyadvertiser.z21.web.core.windows.net
Calculate Final Concentration After Dilution Dilution Factor Solution Chemistry This way of expressing a dilution as a ratio of the parts of solute to the total number of parts. dilution factor is the ratio of the solute to solvent or simply the ratio of the volume of the initial solution to the volume of the. Understand how stock solutions are used. a dilute solution is one in. Dilution Factor Solution Chemistry.
From www.youtube.com
Dilution Problems Chemistry Tutorial YouTube Dilution Factor Solution Chemistry the dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total. How does dilution affect the reaction rate? chemistry solutions dilution calculations. Compute the initial or final concentration. free online dilution calculator. learn how to dilute and concentrate solutions. Use the dilution equation. Dilution Factor Solution Chemistry.
From www.thetechedvocate.org
How to Calculate the Dilution Factor The Tech Edvocate Dilution Factor Solution Chemistry Concentration is the process of. using dilution factors. Understand how stock solutions are used. the solution dilution calculator will calculate for you how to dilute a stock solution of known concentration to obtain. in chemistry and biology, the dilution ratio and dilution factor are two related (but slightly different) expressions of the. the dilution factor represents. Dilution Factor Solution Chemistry.