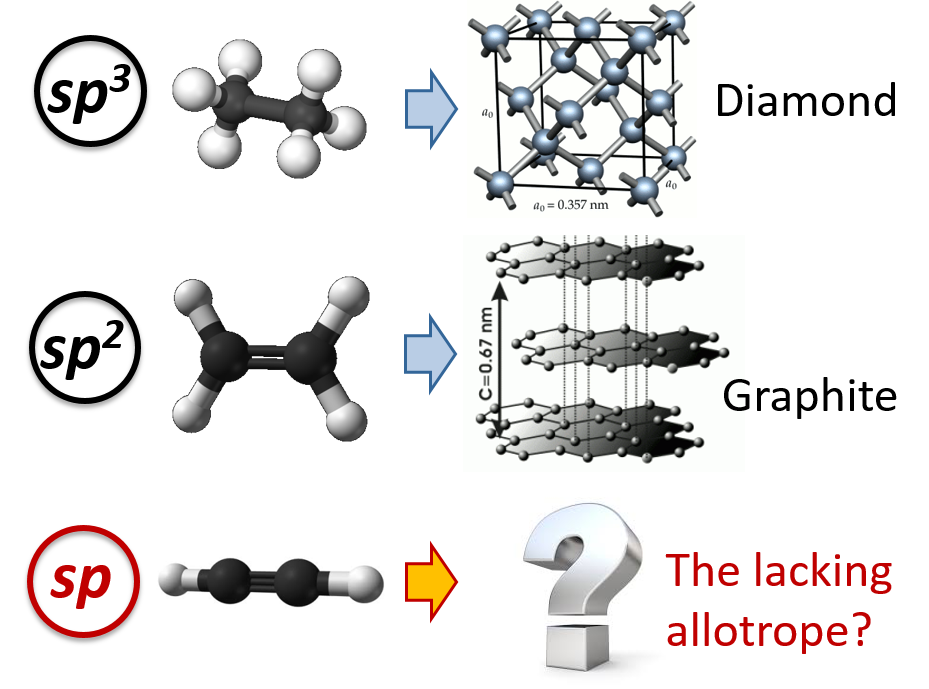
Sp2 Hybridized Carbon Examples . such a variety is due to the ability of carbon to make use of sp, sp 2, and sp 3 hybrid orbitals for the bonding. in sp² hybridization, one s orbital and two p orbitals hybridize to form three sp² orbitals, each consisting of 33% s character and. examples of compounds with sp hybridized carbon atoms include acetylene (c 2 h 2) and carbon monoxide (co). the more electronegative oxygen atom attracts bonding electrons towards itself more strongly than carbon. In the hybrid orbital picture of acetylene, both carbons are sp. The hybrid orbitals are placed in a triangular arrangement with 120° angles between bonds. the sp 2 hybridization is the mixing of one s and two p atomic orbitals, which involves the promotion of one electron in the s orbital. A beryllium hydride \(\left( \ce{beh_2} \right)\) molecule is predicted to be linear by vsepr. three of the four valence electrons on each carbon are distributed to the three sp 2 hybrid orbitals, while the. All the carbon atoms in an alkane are sp3 hybridized with tetrahedral geometry. the best example is the alkanes. any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. the observed structure of the borane molecule, bh 3, suggests sp 2 hybridization for boron in this compound. difference between sp3, sp2, and sp hybrid orbitals based on orbitals involved, images, shape & bond angle, impact of the lone. * each carbon atom undergoes 'sp' hybridization by using a 2s and one 2p orbitals in the excited state to give two.
from www.esplore.polimi.it
The hybrid orbitals are placed in a triangular arrangement with 120° angles between bonds. three of the four valence electrons on each carbon are distributed to the three sp 2 hybrid orbitals, while the. in ethene, the two carbon atoms form a σ bond by overlapping one sp 2 orbital from each carbon atom. There is a formation of two single bonds and one double bond between three atoms. the observed structure of the borane molecule, bh 3, suggests sp 2 hybridization for boron in this compound. the more electronegative oxygen atom attracts bonding electrons towards itself more strongly than carbon. A beryllium hydride \(\left( \ce{beh_2} \right)\) molecule is predicted to be linear by vsepr. One of the \(2s\) electrons is first promoted to the empty \(2p_x\) orbital (see figure below). In the hybrid orbital picture of acetylene, both carbons are sp. * each carbon atom undergoes 'sp' hybridization by using a 2s and one 2p orbitals in the excited state to give two.
What’s linear spCarbon EspLORE
Sp2 Hybridized Carbon Examples the sp 2 hybridization is the mixing of one s and two p atomic orbitals, which involves the promotion of one electron in the s orbital. in sp² hybridization, one s orbital and two p orbitals hybridize to form three sp² orbitals, each consisting of 33% s character and. the more electronegative oxygen atom attracts bonding electrons towards itself more strongly than carbon. an example of carbon with sp^2 hybridized atomic orbital is alkene, specifically the two carbons involved in. There is a formation of two single bonds and one double bond between three atoms. All the carbon atoms in an alkane are sp3 hybridized with tetrahedral geometry. Benzene is a classic example of a compound with sp2 hybridized carbon atoms. the best example is the alkanes. Besides these structures there are more. examples of compounds with sp hybridized carbon atoms include acetylene (c 2 h 2) and carbon monoxide (co). The hybrid orbitals are placed in a triangular arrangement with 120° angles between bonds. One of the \(2s\) electrons is first promoted to the empty \(2p_x\) orbital (see figure below). In the hybrid orbital picture of acetylene, both carbons are sp. any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. such a variety is due to the ability of carbon to make use of sp, sp 2, and sp 3 hybrid orbitals for the bonding. when two sp 2 hybridized carbon atoms approach each other to bond, two sp 2 orbitals approach each other head to head,.
From infinitylearn.com
(Hybridization Of Carbon Dioxide) Infinity Learn Sp2 Hybridized Carbon Examples this lecture is about hybridization of atomic orbitals, pi bonds, sigma bonds and sp, sp2, sp3 hybridization of carbon in. when two sp 2 hybridized carbon atoms approach each other to bond, two sp 2 orbitals approach each other head to head,. the observed structure of the borane molecule, bh 3, suggests sp 2 hybridization for boron. Sp2 Hybridized Carbon Examples.
From www.researchgate.net
Representation of carbon structures hybridization states. Download Sp2 Hybridized Carbon Examples any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. the sp 2 hybridization is the mixing of one s and two p atomic orbitals, which involves the promotion of one electron in the s orbital. in ethene, the two carbon atoms form a σ bond by overlapping. Sp2 Hybridized Carbon Examples.
From www.animalia-life.club
Sp2 Hybridization Shape Sp2 Hybridized Carbon Examples All the carbon atoms in an alkane are sp3 hybridized with tetrahedral geometry. In the hybrid orbital picture of acetylene, both carbons are sp. in ethene, the two carbon atoms form a σ bond by overlapping one sp 2 orbital from each carbon atom. difference between sp3, sp2, and sp hybrid orbitals based on orbitals involved, images, shape. Sp2 Hybridized Carbon Examples.
From mungfali.com
Hybridization Periodic Table Sp2 Hybridized Carbon Examples In the hybrid orbital picture of acetylene, both carbons are sp. in ethene, the two carbon atoms form a σ bond by overlapping one sp 2 orbital from each carbon atom. One of the \(2s\) electrons is first promoted to the empty \(2p_x\) orbital (see figure below). an example of carbon with sp^2 hybridized atomic orbital is alkene,. Sp2 Hybridized Carbon Examples.
From www.chemistrylibrary.org
Hybridization Sp2 Hybridized Carbon Examples A beryllium hydride \(\left( \ce{beh_2} \right)\) molecule is predicted to be linear by vsepr. this lecture is about hybridization of atomic orbitals, pi bonds, sigma bonds and sp, sp2, sp3 hybridization of carbon in. difference between sp3, sp2, and sp hybrid orbitals based on orbitals involved, images, shape & bond angle, impact of the lone. Benzene is a. Sp2 Hybridized Carbon Examples.
From www.animalia-life.club
Sp2 Hybridization Shape Sp2 Hybridized Carbon Examples an example of carbon with sp^2 hybridized atomic orbital is alkene, specifically the two carbons involved in. such a variety is due to the ability of carbon to make use of sp, sp 2, and sp 3 hybrid orbitals for the bonding. three of the four valence electrons on each carbon are distributed to the three sp. Sp2 Hybridized Carbon Examples.
From www.slideserve.com
PPT Covalent Bonding Orbitals Adapted from bobcatchemistry PowerPoint Sp2 Hybridized Carbon Examples in sp² hybridization, one s orbital and two p orbitals hybridize to form three sp² orbitals, each consisting of 33% s character and. Benzene is a classic example of a compound with sp2 hybridized carbon atoms. this lecture is about hybridization of atomic orbitals, pi bonds, sigma bonds and sp, sp2, sp3 hybridization of carbon in. the. Sp2 Hybridized Carbon Examples.
From ar.inspiredpencil.com
Sp2 Hybridization Examples Sp2 Hybridized Carbon Examples The hybrid orbitals are placed in a triangular arrangement with 120° angles between bonds. the sp 2 hybridization is the mixing of one s and two p atomic orbitals, which involves the promotion of one electron in the s orbital. any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp. Sp2 Hybridized Carbon Examples.
From ar.inspiredpencil.com
Sp Hybridization Carbon Sp2 Hybridized Carbon Examples All the carbon atoms in an alkane are sp3 hybridized with tetrahedral geometry. In the hybrid orbital picture of acetylene, both carbons are sp. A beryllium hydride \(\left( \ce{beh_2} \right)\) molecule is predicted to be linear by vsepr. The hybrid orbitals are placed in a triangular arrangement with 120° angles between bonds. the more electronegative oxygen atom attracts bonding. Sp2 Hybridized Carbon Examples.
From courses.lumenlearning.com
Valence Bond Theory and Hybrid Orbitals Introductory Chemistry Sp2 Hybridized Carbon Examples The beryllium atom contains all paired electrons and so must also undergo hybridization. difference between sp3, sp2, and sp hybrid orbitals based on orbitals involved, images, shape & bond angle, impact of the lone. an example of carbon with sp^2 hybridized atomic orbital is alkene, specifically the two carbons involved in. such a variety is due to. Sp2 Hybridized Carbon Examples.
From chem.libretexts.org
Hybridization Chemistry LibreTexts Sp2 Hybridized Carbon Examples the sp hybridization is also called diagonal hybridization. There is a formation of two single bonds and one double bond between three atoms. when two sp 2 hybridized carbon atoms approach each other to bond, two sp 2 orbitals approach each other head to head,. this lecture is about hybridization of atomic orbitals, pi bonds, sigma bonds. Sp2 Hybridized Carbon Examples.
From ar.inspiredpencil.com
Sp Hybridization Carbon Sp2 Hybridized Carbon Examples * each carbon atom undergoes 'sp' hybridization by using a 2s and one 2p orbitals in the excited state to give two. the sp hybridization is also called diagonal hybridization. A beryllium hydride \(\left( \ce{beh_2} \right)\) molecule is predicted to be linear by vsepr. The beryllium atom contains all paired electrons and so must also undergo hybridization. In. Sp2 Hybridized Carbon Examples.
From mungfali.com
Hybridization Periodic Table Sp2 Hybridized Carbon Examples three of the four valence electrons on each carbon are distributed to the three sp 2 hybrid orbitals, while the. the sp hybridization is also called diagonal hybridization. in sp² hybridization, one s orbital and two p orbitals hybridize to form three sp² orbitals, each consisting of 33% s character and. when two sp 2 hybridized. Sp2 Hybridized Carbon Examples.
From brilliant.org
Hybridisation Brilliant Math & Science Wiki Sp2 Hybridized Carbon Examples Besides these structures there are more. One of the \(2s\) electrons is first promoted to the empty \(2p_x\) orbital (see figure below). the sp hybridization is also called diagonal hybridization. this lecture is about hybridization of atomic orbitals, pi bonds, sigma bonds and sp, sp2, sp3 hybridization of carbon in. The beryllium atom contains all paired electrons and. Sp2 Hybridized Carbon Examples.
From www.youtube.com
Sp2 hybridization Chemical bonds Chemistry Khan Academy YouTube Sp2 Hybridized Carbon Examples There is a formation of two single bonds and one double bond between three atoms. this lecture is about hybridization of atomic orbitals, pi bonds, sigma bonds and sp, sp2, sp3 hybridization of carbon in. the sp hybridization is also called diagonal hybridization. difference between sp3, sp2, and sp hybrid orbitals based on orbitals involved, images, shape. Sp2 Hybridized Carbon Examples.
From www.esplore.polimi.it
What’s linear spCarbon EspLORE Sp2 Hybridized Carbon Examples an example of carbon with sp^2 hybridized atomic orbital is alkene, specifically the two carbons involved in. the best example is the alkanes. the sp 2 hybridization is the mixing of one s and two p atomic orbitals, which involves the promotion of one electron in the s orbital. A beryllium hydride \(\left( \ce{beh_2} \right)\) molecule is. Sp2 Hybridized Carbon Examples.
From www.slideserve.com
PPT Covalent Bonding Orbitals Adapted from bobcatchemistry PowerPoint Sp2 Hybridized Carbon Examples the best example is the alkanes. the observed structure of the borane molecule, bh 3, suggests sp 2 hybridization for boron in this compound. All the carbon atoms in an alkane are sp3 hybridized with tetrahedral geometry. such a variety is due to the ability of carbon to make use of sp, sp 2, and sp 3. Sp2 Hybridized Carbon Examples.
From www.slideserve.com
PPT Covalent Bonding Orbitals Adapted from bobcatchemistry PowerPoint Sp2 Hybridized Carbon Examples * each carbon atom undergoes 'sp' hybridization by using a 2s and one 2p orbitals in the excited state to give two. three of the four valence electrons on each carbon are distributed to the three sp 2 hybrid orbitals, while the. such a variety is due to the ability of carbon to make use of sp,. Sp2 Hybridized Carbon Examples.
From byjus.com
Hybridization of Carbon Molecular Geometry and Bond Angles Sp2 Hybridized Carbon Examples Besides these structures there are more. the observed structure of the borane molecule, bh 3, suggests sp 2 hybridization for boron in this compound. this lecture is about hybridization of atomic orbitals, pi bonds, sigma bonds and sp, sp2, sp3 hybridization of carbon in. One of the \(2s\) electrons is first promoted to the empty \(2p_x\) orbital (see. Sp2 Hybridized Carbon Examples.
From www.coursehero.com
[Solved] Identify the type of hybridization (sp, sp2, sp3) for all C Sp2 Hybridized Carbon Examples when two sp 2 hybridized carbon atoms approach each other to bond, two sp 2 orbitals approach each other head to head,. The beryllium atom contains all paired electrons and so must also undergo hybridization. such a variety is due to the ability of carbon to make use of sp, sp 2, and sp 3 hybrid orbitals for. Sp2 Hybridized Carbon Examples.
From socratic.org
Identify the hybridization of the three carbon atoms located in the Sp2 Hybridized Carbon Examples any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization. One of the \(2s\) electrons is first promoted to the empty \(2p_x\) orbital (see figure below). three of the four valence electrons on each carbon are distributed to the three sp 2 hybrid orbitals, while the. A beryllium hydride. Sp2 Hybridized Carbon Examples.
From studybreathings.z21.web.core.windows.net
How To Calculate Sp Sp2 Hybridized Carbon Examples an example of carbon with sp^2 hybridized atomic orbital is alkene, specifically the two carbons involved in. All the carbon atoms in an alkane are sp3 hybridized with tetrahedral geometry. the sp hybridization is also called diagonal hybridization. * each carbon atom undergoes 'sp' hybridization by using a 2s and one 2p orbitals in the excited state. Sp2 Hybridized Carbon Examples.
From www.youtube.com
CO2 Hybridization Hybrid Orbitals for CO2 YouTube Sp2 Hybridized Carbon Examples this lecture is about hybridization of atomic orbitals, pi bonds, sigma bonds and sp, sp2, sp3 hybridization of carbon in. when two sp 2 hybridized carbon atoms approach each other to bond, two sp 2 orbitals approach each other head to head,. All the carbon atoms in an alkane are sp3 hybridized with tetrahedral geometry. in ethene,. Sp2 Hybridized Carbon Examples.
From www.chegg.com
Solved All six carbon atoms are sp2 hybridized. CLIC Sp2 Hybridized Carbon Examples The hybrid orbitals are placed in a triangular arrangement with 120° angles between bonds. examples of compounds with sp hybridized carbon atoms include acetylene (c 2 h 2) and carbon monoxide (co). In the hybrid orbital picture of acetylene, both carbons are sp. three of the four valence electrons on each carbon are distributed to the three sp. Sp2 Hybridized Carbon Examples.
From www.solutionspile.com
[Solved] How many sp2 hybridized carbon atoms are in this s Sp2 Hybridized Carbon Examples the more electronegative oxygen atom attracts bonding electrons towards itself more strongly than carbon. All the carbon atoms in an alkane are sp3 hybridized with tetrahedral geometry. In the hybrid orbital picture of acetylene, both carbons are sp. The hybrid orbitals are placed in a triangular arrangement with 120° angles between bonds. Besides these structures there are more. . Sp2 Hybridized Carbon Examples.
From www.youtube.com
Hybridization of Atomic Orbitals Sigma & Pi Bonds Sp, Sp2, Sp3 Sp2 Hybridized Carbon Examples the observed structure of the borane molecule, bh 3, suggests sp 2 hybridization for boron in this compound. three of the four valence electrons on each carbon are distributed to the three sp 2 hybrid orbitals, while the. in sp² hybridization, one s orbital and two p orbitals hybridize to form three sp² orbitals, each consisting of. Sp2 Hybridized Carbon Examples.
From courses.lumenlearning.com
2.2. Hybrid orbitals Organic Chemistry 1 An open textbook Sp2 Hybridized Carbon Examples when two sp 2 hybridized carbon atoms approach each other to bond, two sp 2 orbitals approach each other head to head,. this lecture is about hybridization of atomic orbitals, pi bonds, sigma bonds and sp, sp2, sp3 hybridization of carbon in. any central atom surrounded by just two regions of valence electron density in a molecule. Sp2 Hybridized Carbon Examples.
From www.youtube.com
14.2/S2.2 .16 Lewis, hybridization (sp3,sp2,sp) , shapes and angles [HL Sp2 Hybridized Carbon Examples an example of carbon with sp^2 hybridized atomic orbital is alkene, specifically the two carbons involved in. in ethene, the two carbon atoms form a σ bond by overlapping one sp 2 orbital from each carbon atom. this lecture is about hybridization of atomic orbitals, pi bonds, sigma bonds and sp, sp2, sp3 hybridization of carbon in.. Sp2 Hybridized Carbon Examples.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry sp2 orbital Sp2 Hybridized Carbon Examples A beryllium hydride \(\left( \ce{beh_2} \right)\) molecule is predicted to be linear by vsepr. In the hybrid orbital picture of acetylene, both carbons are sp. in ethene, the two carbon atoms form a σ bond by overlapping one sp 2 orbital from each carbon atom. when two sp 2 hybridized carbon atoms approach each other to bond, two. Sp2 Hybridized Carbon Examples.
From formic-acid.ir
میانبر استاد شیمی آلی تولیدی فرمیک Sp2 Hybridized Carbon Examples difference between sp3, sp2, and sp hybrid orbitals based on orbitals involved, images, shape & bond angle, impact of the lone. an example of carbon with sp^2 hybridized atomic orbital is alkene, specifically the two carbons involved in. One of the \(2s\) electrons is first promoted to the empty \(2p_x\) orbital (see figure below). The beryllium atom contains. Sp2 Hybridized Carbon Examples.
From www.youtube.com
How to identify hybridization of carbon atom sp sp2 sp3 YouTube Sp2 Hybridized Carbon Examples the best example is the alkanes. this lecture is about hybridization of atomic orbitals, pi bonds, sigma bonds and sp, sp2, sp3 hybridization of carbon in. The hybrid orbitals are placed in a triangular arrangement with 120° angles between bonds. three of the four valence electrons on each carbon are distributed to the three sp 2 hybrid. Sp2 Hybridized Carbon Examples.
From ar.inspiredpencil.com
Sp2 Hybridization Examples Sp2 Hybridized Carbon Examples The hybrid orbitals are placed in a triangular arrangement with 120° angles between bonds. There is a formation of two single bonds and one double bond between three atoms. in sp² hybridization, one s orbital and two p orbitals hybridize to form three sp² orbitals, each consisting of 33% s character and. the observed structure of the borane. Sp2 Hybridized Carbon Examples.
From courses.lumenlearning.com
13.2. Molecular orbitals for ethene Organic Chemistry II Sp2 Hybridized Carbon Examples the sp 2 hybridization is the mixing of one s and two p atomic orbitals, which involves the promotion of one electron in the s orbital. in ethene, the two carbon atoms form a σ bond by overlapping one sp 2 orbital from each carbon atom. the sp hybridization is also called diagonal hybridization. any central. Sp2 Hybridized Carbon Examples.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry sp2 orbital Sp2 Hybridized Carbon Examples The hybrid orbitals are placed in a triangular arrangement with 120° angles between bonds. the best example is the alkanes. examples of compounds with sp hybridized carbon atoms include acetylene (c 2 h 2) and carbon monoxide (co). Benzene is a classic example of a compound with sp2 hybridized carbon atoms. such a variety is due to. Sp2 Hybridized Carbon Examples.
From www.toppr.com
Which of the following represents the given sequence of hybridisation Sp2 Hybridized Carbon Examples the sp 2 hybridization is the mixing of one s and two p atomic orbitals, which involves the promotion of one electron in the s orbital. the more electronegative oxygen atom attracts bonding electrons towards itself more strongly than carbon. in ethene, the two carbon atoms form a σ bond by overlapping one sp 2 orbital from. Sp2 Hybridized Carbon Examples.