What Is Calorimetry And Its Principle . Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer. The principle of calorimetry signifies the “law of conservation of energy.” hence, this statement means that the total amount of heat absorbed. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Chemists use calorimetry to determine the. By knowing the change in the heat we can determine whether or. Calorimetry is the process of measuring the amount of heat absorbed or released during a chemical reaction. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.
from www.slideserve.com
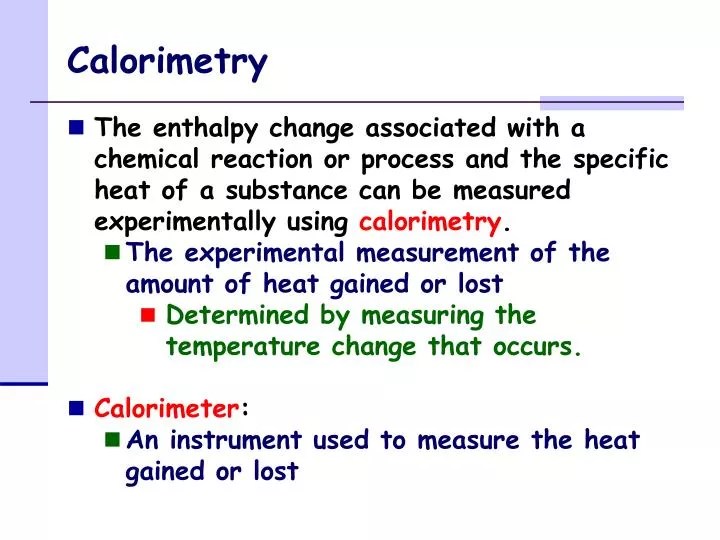
Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. By knowing the change in the heat we can determine whether or. The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. The principle of calorimetry signifies the “law of conservation of energy.” hence, this statement means that the total amount of heat absorbed. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. Chemists use calorimetry to determine the. Calorimetry is the process of measuring the amount of heat absorbed or released during a chemical reaction. For example, when an exothermic reaction occurs in solution in a calorimeter, the.
PPT Calorimetry PowerPoint Presentation, free download ID6133898
What Is Calorimetry And Its Principle Chemists use calorimetry to determine the. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is the process of measuring the amount of heat absorbed or released during a chemical reaction. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. Chemists use calorimetry to determine the. By knowing the change in the heat we can determine whether or. The principle of calorimetry signifies the “law of conservation of energy.” hence, this statement means that the total amount of heat absorbed. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction.
From www.youtube.com
CALORIMETRY Its Principle & Applications with Numericals Class 11 What Is Calorimetry And Its Principle Calorimetry is the process of measuring the amount of heat absorbed or released during a chemical reaction. Chemists use calorimetry to determine the. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. For example, when an exothermic reaction occurs in solution in a calorimeter, the. The principle of calorimetry signifies. What Is Calorimetry And Its Principle.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types What Is Calorimetry And Its Principle The principle of calorimetry signifies the “law of conservation of energy.” hence, this statement means that the total amount of heat absorbed. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. By knowing the change in the heat we can determine whether or. Calorimetry is a branch of science concerned. What Is Calorimetry And Its Principle.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 What Is Calorimetry And Its Principle The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is a branch of science concerned with. What Is Calorimetry And Its Principle.
From www.slideserve.com
PPT CALORIMETRY PowerPoint Presentation, free download ID5767247 What Is Calorimetry And Its Principle The principle of calorimetry signifies the “law of conservation of energy.” hence, this statement means that the total amount of heat absorbed. By knowing the change in the heat we can determine whether or. The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer. A calorimeter is a. What Is Calorimetry And Its Principle.
From joiwmxbys.blob.core.windows.net
Calorimetry With Two Solutions at Angelica Kirkland blog What Is Calorimetry And Its Principle For example, when an exothermic reaction occurs in solution in a calorimeter, the. The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer. Chemists use calorimetry to determine the. By knowing the change in the heat we can determine whether or. Calorimetry is the process of measuring the. What Is Calorimetry And Its Principle.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID1875569 What Is Calorimetry And Its Principle Calorimetry is the process of measuring the amount of heat absorbed or released during a chemical reaction. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. Chemists use. What Is Calorimetry And Its Principle.
From www.youtube.com
050 Calorimetry YouTube What Is Calorimetry And Its Principle Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. The principle of calorimetry signifies the “law of conservation of energy.” hence, this statement means that the total amount of heat absorbed. For example, when an exothermic reaction occurs in solution in a calorimeter, the. By knowing the change in the. What Is Calorimetry And Its Principle.
From study.com
Calorimetry Definition, Equation & Types Lesson What Is Calorimetry And Its Principle Chemists use calorimetry to determine the. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the process of measuring the amount of heat absorbed or released during a chemical reaction. For example, when an exothermic reaction occurs in solution in a calorimeter, the. The principle of calorimetry signifies. What Is Calorimetry And Its Principle.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson What Is Calorimetry And Its Principle Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. The act. What Is Calorimetry And Its Principle.
From revisionug.com
What is Calorimetry? What Is Calorimetry And Its Principle For example, when an exothermic reaction occurs in solution in a calorimeter, the. The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer. Calorimetry is the process of measuring the amount of heat absorbed or released during a chemical reaction. Calorimetry is the process of measuring the amount. What Is Calorimetry And Its Principle.
From www.youtube.com
Principle of Calorimetry YouTube What Is Calorimetry And Its Principle The principle of calorimetry signifies the “law of conservation of energy.” hence, this statement means that the total amount of heat absorbed. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. By knowing. What Is Calorimetry And Its Principle.
From www.youtube.com
CALORIMETRY Principle of Calorimetry, Calorimeter, it's application What Is Calorimetry And Its Principle Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. For example, when an exothermic reaction occurs in solution in a calorimeter, the. The principle of calorimetry signifies the “law of conservation of energy.” hence, this statement means that the total amount of heat absorbed. Chemists use calorimetry to determine the. By knowing. What Is Calorimetry And Its Principle.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals What Is Calorimetry And Its Principle Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Chemists use calorimetry to determine the. By knowing the change in the heat we can determine whether or. Calorimetry is the process. What Is Calorimetry And Its Principle.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 What Is Calorimetry And Its Principle A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer. Calorimetry is the process of measuring the amount of heat absorbed or released during a chemical reaction. Calorimetry is. What Is Calorimetry And Its Principle.
From saylordotorg.github.io
Calorimetry What Is Calorimetry And Its Principle The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. By knowing the change in the heat we can determine whether or. A calorimeter is a. What Is Calorimetry And Its Principle.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle What Is Calorimetry And Its Principle Calorimetry is the process of measuring the amount of heat absorbed or released during a chemical reaction. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Chemists use calorimetry to determine the. A calorimeter. What Is Calorimetry And Its Principle.
From www.researchgate.net
Principle of calorimetry. Download Scientific Diagram What Is Calorimetry And Its Principle By knowing the change in the heat we can determine whether or. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. Chemists use calorimetry to determine the. The act or science of measuring the changes in the state variables of a body in order to calculate the. What Is Calorimetry And Its Principle.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3850751 What Is Calorimetry And Its Principle Calorimetry is the process of measuring the amount of heat absorbed or released during a chemical reaction. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. The principle of calorimetry signifies the “law of conservation of energy.” hence, this statement means that the total amount of heat absorbed. A calorimeter is a. What Is Calorimetry And Its Principle.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube What Is Calorimetry And Its Principle For example, when an exothermic reaction occurs in solution in a calorimeter, the. The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer. Calorimetry is the process of measuring the amount of heat absorbed or released during a chemical reaction. Chemists use calorimetry to determine the. Calorimetry is. What Is Calorimetry And Its Principle.
From byjus.com
State the Principle of calorimetry What Is Calorimetry And Its Principle A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. The principle of calorimetry signifies the “law of conservation of energy.” hence, this statement means that the total amount. What Is Calorimetry And Its Principle.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry What Is Calorimetry And Its Principle Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. By knowing the change in. What Is Calorimetry And Its Principle.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii What Is Calorimetry And Its Principle The principle of calorimetry signifies the “law of conservation of energy.” hence, this statement means that the total amount of heat absorbed. By knowing the change in the heat we can determine whether or. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is the process of measuring the amount of. What Is Calorimetry And Its Principle.
From joiddcrwa.blob.core.windows.net
What Is Calorimetry Example at Donovan blog What Is Calorimetry And Its Principle The principle of calorimetry signifies the “law of conservation of energy.” hence, this statement means that the total amount of heat absorbed. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. Calorimetry is the process of measuring the amount of heat absorbed or released during a chemical. What Is Calorimetry And Its Principle.
From www.thoughtco.com
Calorimeter Definition in Chemistry What Is Calorimetry And Its Principle Calorimetry is the process of measuring the amount of heat absorbed or released during a chemical reaction. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry is a branch of science concerned with measuring a body’s. What Is Calorimetry And Its Principle.
From www.slideserve.com
PPT Energy Changes in Reactions PowerPoint Presentation, free What Is Calorimetry And Its Principle The principle of calorimetry signifies the “law of conservation of energy.” hence, this statement means that the total amount of heat absorbed. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. For example, when an exothermic reaction occurs in solution in a calorimeter, the. By knowing the change in the. What Is Calorimetry And Its Principle.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID1084959 What Is Calorimetry And Its Principle Calorimetry is the process of measuring the amount of heat absorbed or released during a chemical reaction. The principle of calorimetry signifies the “law of conservation of energy.” hence, this statement means that the total amount of heat absorbed. Chemists use calorimetry to determine the. For example, when an exothermic reaction occurs in solution in a calorimeter, the. A calorimeter. What Is Calorimetry And Its Principle.
From sites.google.com
Calorimetry Preliminary HSC Chemistry What Is Calorimetry And Its Principle Chemists use calorimetry to determine the. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a. What Is Calorimetry And Its Principle.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses What Is Calorimetry And Its Principle Chemists use calorimetry to determine the. For example, when an exothermic reaction occurs in solution in a calorimeter, the. The principle of calorimetry signifies the “law of conservation of energy.” hence, this statement means that the total amount of heat absorbed. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.. What Is Calorimetry And Its Principle.
From courses.lumenlearning.com
Calorimetry Chemistry I What Is Calorimetry And Its Principle The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer. Calorimetry is the process of measuring the amount of heat absorbed or released during a chemical reaction. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The principle. What Is Calorimetry And Its Principle.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica What Is Calorimetry And Its Principle The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer. The principle of calorimetry signifies the “law of conservation of energy.” hence, this statement means that the total amount of heat absorbed. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical. What Is Calorimetry And Its Principle.
From studylib.net
Calorimetry What Is Calorimetry And Its Principle The principle of calorimetry signifies the “law of conservation of energy.” hence, this statement means that the total amount of heat absorbed. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Calorimetry is the process of measuring the amount of heat absorbed or released during a chemical reaction. Chemists use calorimetry to determine the. Calorimetry is. What Is Calorimetry And Its Principle.
From www.slideserve.com
PPT Thermochemistry Notes PowerPoint Presentation, free download ID What Is Calorimetry And Its Principle For example, when an exothermic reaction occurs in solution in a calorimeter, the. The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The principle of calorimetry signifies the. What Is Calorimetry And Its Principle.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors What Is Calorimetry And Its Principle Chemists use calorimetry to determine the. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. What Is Calorimetry And Its Principle.
From scienceinfo.com
Calorimetry Definition, Principle, Types, Application, and Limitations What Is Calorimetry And Its Principle Calorimetry is the process of measuring the amount of heat absorbed or released during a chemical reaction. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. The act or science of measuring the changes in the state variables of a body in order to calculate the heat transfer. For example, when an. What Is Calorimetry And Its Principle.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID1551165 What Is Calorimetry And Its Principle Chemists use calorimetry to determine the. For example, when an exothermic reaction occurs in solution in a calorimeter, the. By knowing the change in the heat we can determine whether or. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. The principle of calorimetry signifies the “law. What Is Calorimetry And Its Principle.