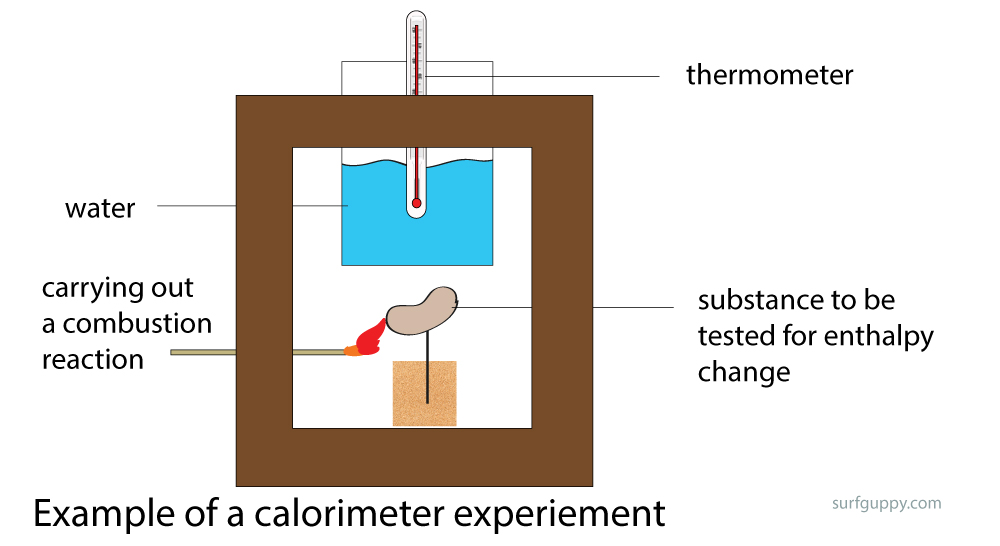
What Exactly Does A Calorimeter Measure . Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Chemists use calorimetry to determine the. After the calorimeter calibration, the chemist will already have a. Calorimetry is the field of science that deals with the measurement of the state of a body with respect to the thermal aspects in order to examine its physical and chemical changes. Technically, this is called an adiabatic surface in that no heat flows in and out of the calorimeter, which is in contrast to isothermal, which means constant temperature. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the. Basically, a calorimeter measures the change in temperature of the calorimeter and its contents.
from surfguppy.com
Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. After the calorimeter calibration, the chemist will already have a. Chemists use calorimetry to determine the. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Calorimetry is the field of science that deals with the measurement of the state of a body with respect to the thermal aspects in order to examine its physical and chemical changes. Technically, this is called an adiabatic surface in that no heat flows in and out of the calorimeter, which is in contrast to isothermal, which means constant temperature. Basically, a calorimeter measures the change in temperature of the calorimeter and its contents.
Enthalpy Surfguppy Chemistry made easy visual learning
What Exactly Does A Calorimeter Measure Technically, this is called an adiabatic surface in that no heat flows in and out of the calorimeter, which is in contrast to isothermal, which means constant temperature. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the. Technically, this is called an adiabatic surface in that no heat flows in and out of the calorimeter, which is in contrast to isothermal, which means constant temperature. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Basically, a calorimeter measures the change in temperature of the calorimeter and its contents. Calorimetry is the field of science that deals with the measurement of the state of a body with respect to the thermal aspects in order to examine its physical and chemical changes. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. After the calorimeter calibration, the chemist will already have a. Chemists use calorimetry to determine the. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID9276632 What Exactly Does A Calorimeter Measure Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Calorimetry is the field of science that deals with the measurement of the state of a body with respect to the thermal aspects in order to examine its physical and chemical changes. A calorimeter is an instrument that has a. What Exactly Does A Calorimeter Measure.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors What Exactly Does A Calorimeter Measure Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Technically, this is called an adiabatic surface in that no heat flows in and out of the calorimeter, which is in contrast to isothermal, which means constant temperature. Calorimetry is the field of science that deals with the measurement of. What Exactly Does A Calorimeter Measure.
From www.scienceabc.com
How Do We Calculate How Many Carbs Are In The Food? What Exactly Does A Calorimeter Measure Calorimetry is the field of science that deals with the measurement of the state of a body with respect to the thermal aspects in order to examine its physical and chemical changes. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the. Chemists use calorimetry. What Exactly Does A Calorimeter Measure.
From courses.lumenlearning.com
Calorimetry Introductory Chemistry Lecture & Lab What Exactly Does A Calorimeter Measure A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. After the calorimeter calibration, the chemist will already have a. Chemists use calorimetry to determine the. Calorimetry is the. What Exactly Does A Calorimeter Measure.
From faqguide.co
What does a calorimeter do? Explained by FAQGuide What Exactly Does A Calorimeter Measure Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the. After the calorimeter calibration, the chemist will already have a. Calorimetry is a field of. What Exactly Does A Calorimeter Measure.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses What Exactly Does A Calorimeter Measure Basically, a calorimeter measures the change in temperature of the calorimeter and its contents. Calorimetry is the field of science that deals with the measurement of the state of a body with respect to the thermal aspects in order to examine its physical and chemical changes. Chemists use calorimetry to determine the. Technically, this is called an adiabatic surface in. What Exactly Does A Calorimeter Measure.
From www.youtube.com
Principle of Calorimetry YouTube What Exactly Does A Calorimeter Measure Chemists use calorimetry to determine the. Calorimetry is the field of science that deals with the measurement of the state of a body with respect to the thermal aspects in order to examine its physical and chemical changes. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Calorimetry. What Exactly Does A Calorimeter Measure.
From physique.ensc-rennes.fr
TP calorimetry What Exactly Does A Calorimeter Measure Basically, a calorimeter measures the change in temperature of the calorimeter and its contents. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Technically, this is called an adiabatic surface. What Exactly Does A Calorimeter Measure.
From users.highland.edu
Calorimetry What Exactly Does A Calorimeter Measure Technically, this is called an adiabatic surface in that no heat flows in and out of the calorimeter, which is in contrast to isothermal, which means constant temperature. After the calorimeter calibration, the chemist will already have a. Calorimetry is the field of science that deals with the measurement of the state of a body with respect to the thermal. What Exactly Does A Calorimeter Measure.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube What Exactly Does A Calorimeter Measure Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Technically, this is called an adiabatic surface in that no heat flows in and out of the calorimeter, which is in. What Exactly Does A Calorimeter Measure.
From www.animalia-life.club
Calorimeter Diagram What Exactly Does A Calorimeter Measure Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. After the calorimeter calibration, the chemist will already have a. Chemists use calorimetry to determine the. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Basically, a calorimeter measures the. What Exactly Does A Calorimeter Measure.
From slideplayer.com
A “Calorimeter”. Calorimetry Calculations When analyzing data obtained What Exactly Does A Calorimeter Measure A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. What Exactly Does A Calorimeter Measure.
From www.youtube.com
Thermochemistry Enthalpy and Coffee Cup Calorimeter. YouTube What Exactly Does A Calorimeter Measure Basically, a calorimeter measures the change in temperature of the calorimeter and its contents. After the calorimeter calibration, the chemist will already have a. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a. What Exactly Does A Calorimeter Measure.
From www.shaalaa.com
Explain the construction of a calorimeter. Draw the necessary figure What Exactly Does A Calorimeter Measure A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the. Basically, a calorimeter measures the change in temperature of the calorimeter and its contents. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A. What Exactly Does A Calorimeter Measure.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec What Exactly Does A Calorimeter Measure A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. After the calorimeter calibration, the chemist will already have a. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the. Basically, a calorimeter measures. What Exactly Does A Calorimeter Measure.
From www.wisegeek.com
What is Calorimetry? (with pictures) What Exactly Does A Calorimeter Measure A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the. Calorimetry is the field of science that deals with the measurement of the state. What Exactly Does A Calorimeter Measure.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry What Exactly Does A Calorimeter Measure Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Basically, a calorimeter measures the change in temperature of the calorimeter and its contents. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. After the calorimeter calibration, the chemist will. What Exactly Does A Calorimeter Measure.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts What Exactly Does A Calorimeter Measure A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. After the calorimeter calibration, the chemist will already have a. Basically, a calorimeter measures the change in temperature of the calorimeter and its contents. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and. What Exactly Does A Calorimeter Measure.
From courses.lumenlearning.com
Calorimetry CHEM 1305 General Chemistry I—Lecture What Exactly Does A Calorimeter Measure Technically, this is called an adiabatic surface in that no heat flows in and out of the calorimeter, which is in contrast to isothermal, which means constant temperature. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the. Chemists use calorimetry to determine the. Basically,. What Exactly Does A Calorimeter Measure.
From www.thoughtco.com
Calorimeter Definition in Chemistry What Exactly Does A Calorimeter Measure Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the. Basically, a calorimeter measures the change in temperature of the calorimeter and its contents. After the. What Exactly Does A Calorimeter Measure.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation What Exactly Does A Calorimeter Measure A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Basically, a calorimeter measures the change in temperature of the calorimeter and its contents. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. A calorimeter measures the mass of. What Exactly Does A Calorimeter Measure.
From shaunmwilliams.com
Chapter 6 Presentation What Exactly Does A Calorimeter Measure Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the. A calorimeter is an instrument that has a thermally isolated compartment that is designed to. What Exactly Does A Calorimeter Measure.
From mechasource.blogspot.com
An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas What Exactly Does A Calorimeter Measure After the calorimeter calibration, the chemist will already have a. Calorimetry is the field of science that deals with the measurement of the state of a body with respect to the thermal aspects in order to examine its physical and chemical changes. Technically, this is called an adiabatic surface in that no heat flows in and out of the calorimeter,. What Exactly Does A Calorimeter Measure.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download What Exactly Does A Calorimeter Measure After the calorimeter calibration, the chemist will already have a. Technically, this is called an adiabatic surface in that no heat flows in and out of the calorimeter, which is in contrast to isothermal, which means constant temperature. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or. What Exactly Does A Calorimeter Measure.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 What Exactly Does A Calorimeter Measure After the calorimeter calibration, the chemist will already have a. Technically, this is called an adiabatic surface in that no heat flows in and out of the calorimeter, which is in contrast to isothermal, which means constant temperature. Chemists use calorimetry to determine the. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine. What Exactly Does A Calorimeter Measure.
From library.achievingthedream.org
Calorimetry Introductory Chemistry Lecture & Lab What Exactly Does A Calorimeter Measure After the calorimeter calibration, the chemist will already have a. Chemists use calorimetry to determine the. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Calorimetry is the field of science that deals with the measurement of the state of a body with respect to the thermal aspects in. What Exactly Does A Calorimeter Measure.
From manueldesnhray.blogspot.com
Identify What a Coffee Cup Calorimeter Measures. What Exactly Does A Calorimeter Measure After the calorimeter calibration, the chemist will already have a. Basically, a calorimeter measures the change in temperature of the calorimeter and its contents. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the. Calorimeter, device for measuring the heat developed during a mechanical, electrical,. What Exactly Does A Calorimeter Measure.
From chem.libretexts.org
5.3 Calorimetry Chemistry LibreTexts What Exactly Does A Calorimeter Measure A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the. Basically, a calorimeter measures the change in temperature of the calorimeter and its contents. Chemists use calorimetry to determine the. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a. What Exactly Does A Calorimeter Measure.
From www.thermal-engineering.org
How does a calorimeter measure heat What Exactly Does A Calorimeter Measure Calorimetry is the field of science that deals with the measurement of the state of a body with respect to the thermal aspects in order to examine its physical and chemical changes. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Calorimetry is a field of thermochemistry that. What Exactly Does A Calorimeter Measure.
From surfguppy.com
Enthalpy Surfguppy Chemistry made easy visual learning What Exactly Does A Calorimeter Measure Calorimetry is the field of science that deals with the measurement of the state of a body with respect to the thermal aspects in order to examine its physical and chemical changes. Basically, a calorimeter measures the change in temperature of the calorimeter and its contents. A calorimeter is an instrument that has a thermally isolated compartment that is designed. What Exactly Does A Calorimeter Measure.
From www.youtube.com
050 Calorimetry YouTube What Exactly Does A Calorimeter Measure Technically, this is called an adiabatic surface in that no heat flows in and out of the calorimeter, which is in contrast to isothermal, which means constant temperature. Basically, a calorimeter measures the change in temperature of the calorimeter and its contents. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. What Exactly Does A Calorimeter Measure.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica What Exactly Does A Calorimeter Measure Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry is the field of science that deals with the measurement of the state of a body with respect to the thermal aspects in order to examine its physical and chemical changes. Chemists use calorimetry to determine the. A calorimeter is. What Exactly Does A Calorimeter Measure.
From www.freeastroscience.com
Exploring Calorimetry A Journey into Heat Transfer Measurement What Exactly Does A Calorimeter Measure Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Calorimetry is the field of science that deals with the measurement of the state of a body with respect to the thermal aspects in order to examine its physical and chemical changes. Technically, this is called an adiabatic surface in. What Exactly Does A Calorimeter Measure.
From www.youtube.com
Thermochemistry ConstantVolume Calorimeter (Bomb Calorimeter). YouTube What Exactly Does A Calorimeter Measure Technically, this is called an adiabatic surface in that no heat flows in and out of the calorimeter, which is in contrast to isothermal, which means constant temperature. Chemists use calorimetry to determine the. After the calorimeter calibration, the chemist will already have a. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent. What Exactly Does A Calorimeter Measure.
From people.chem.umass.edu
to Adobe GoLive 6 What Exactly Does A Calorimeter Measure Basically, a calorimeter measures the change in temperature of the calorimeter and its contents. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings.. What Exactly Does A Calorimeter Measure.