What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al . The electrons in titanium are arranged in their orbitals as shown. Four electrons in a p subshell. We describe an electron configuration with a symbol that contains three pieces of information (figure 6.25): From the orbital diagram, we can write the electron configuration in an abbreviated form in which the occupied orbitals are identified by their principal quantum number. What is the electron configuration of: This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic. The diagram of an electron configuration specifies the subshell (n and l value, with letter symbol) and. That is, we follow the three important rules:
from stock.adobe.com
The diagram of an electron configuration specifies the subshell (n and l value, with letter symbol) and. We describe an electron configuration with a symbol that contains three pieces of information (figure 6.25): Four electrons in a p subshell. The electrons in titanium are arranged in their orbitals as shown. What is the electron configuration of: This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic. From the orbital diagram, we can write the electron configuration in an abbreviated form in which the occupied orbitals are identified by their principal quantum number. That is, we follow the three important rules:
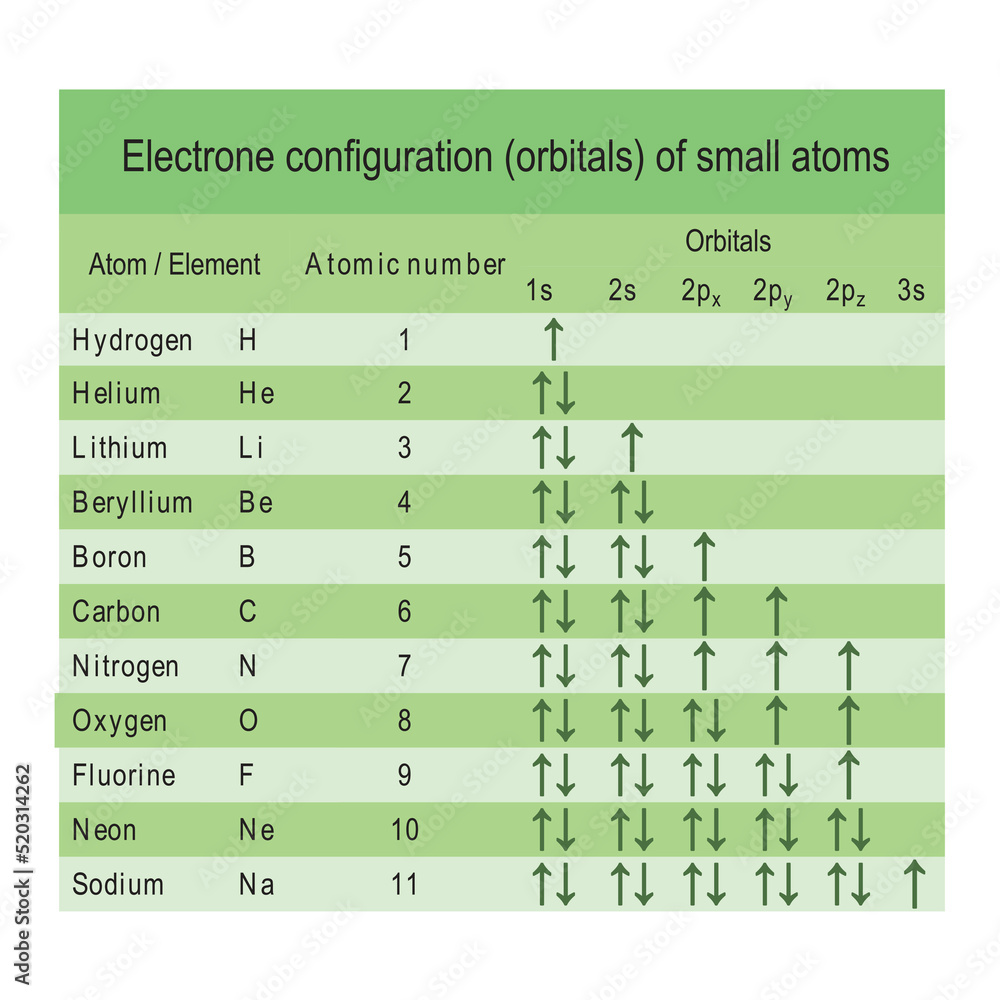
Table showing electron orbital configuration of the smallest atoms
What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic. From the orbital diagram, we can write the electron configuration in an abbreviated form in which the occupied orbitals are identified by their principal quantum number. What is the electron configuration of: The electrons in titanium are arranged in their orbitals as shown. We describe an electron configuration with a symbol that contains three pieces of information (figure 6.25): Four electrons in a p subshell. That is, we follow the three important rules: The diagram of an electron configuration specifies the subshell (n and l value, with letter symbol) and. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic.
From mungfali.com
How Many Electrons Are In Each Shell What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al That is, we follow the three important rules: This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic. The diagram of an electron configuration specifies the subshell (n and l value, with letter symbol) and. We describe an electron configuration with a symbol that contains three pieces of information (figure 6.25): What. What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al.
From general.chemistrysteps.com
Electron Configurations of Ions Chemistry Steps What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al From the orbital diagram, we can write the electron configuration in an abbreviated form in which the occupied orbitals are identified by their principal quantum number. The electrons in titanium are arranged in their orbitals as shown. Four electrons in a p subshell. That is, we follow the three important rules: What is the electron configuration of: This electron configuration. What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al.
From www.sciencefacts.net
Electron Configuration Definition, Examples, Chart, and Diagram What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al We describe an electron configuration with a symbol that contains three pieces of information (figure 6.25): From the orbital diagram, we can write the electron configuration in an abbreviated form in which the occupied orbitals are identified by their principal quantum number. The diagram of an electron configuration specifies the subshell (n and l value, with letter symbol) and. Four. What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al.
From gzscienceclassonline.weebly.com
1. Electron Configuration What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al That is, we follow the three important rules: This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic. What is the electron configuration of: The electrons in titanium are arranged in their orbitals as shown. The diagram of an electron configuration specifies the subshell (n and l value, with letter symbol) and.. What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al.
From www.youtube.com
Lec14 Electron configurations of Ions and Exceptions YouTube What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al What is the electron configuration of: That is, we follow the three important rules: From the orbital diagram, we can write the electron configuration in an abbreviated form in which the occupied orbitals are identified by their principal quantum number. We describe an electron configuration with a symbol that contains three pieces of information (figure 6.25): Four electrons in a. What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al.
From ecurrencythailand.com
What Is The Electron Configuration Of The Mg2+ Cation? All Answers What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al The electrons in titanium are arranged in their orbitals as shown. Four electrons in a p subshell. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic. That is, we follow the three important rules: The diagram of an electron configuration specifies the subshell (n and l value, with letter symbol) and.. What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al.
From general.chemistrysteps.com
Electron Configurations of Ions Chemistry Steps What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al We describe an electron configuration with a symbol that contains three pieces of information (figure 6.25): The electrons in titanium are arranged in their orbitals as shown. The diagram of an electron configuration specifies the subshell (n and l value, with letter symbol) and. What is the electron configuration of: Four electrons in a p subshell. That is, we follow. What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al.
From alevelchemistry.co.uk
Electron Structure ALevel Chemistry Revision Notes What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al What is the electron configuration of: This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic. That is, we follow the three important rules: We describe an electron configuration with a symbol that contains three pieces of information (figure 6.25): Four electrons in a p subshell. The electrons in titanium are arranged. What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al.
From spmchemistry.blog.onlinetuition.com.my
Electron Arrangement in Atom SPM Chemistry What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al That is, we follow the three important rules: The electrons in titanium are arranged in their orbitals as shown. Four electrons in a p subshell. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic. What is the electron configuration of: From the orbital diagram, we can write the electron configuration in. What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al.
From www.britannica.com
Electron shell Definition & Facts Britannica What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al We describe an electron configuration with a symbol that contains three pieces of information (figure 6.25): The diagram of an electron configuration specifies the subshell (n and l value, with letter symbol) and. That is, we follow the three important rules: From the orbital diagram, we can write the electron configuration in an abbreviated form in which the occupied orbitals. What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al.
From www.youtube.com
CHEMISTRY 101 Electron configurations for ions YouTube What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al The diagram of an electron configuration specifies the subshell (n and l value, with letter symbol) and. We describe an electron configuration with a symbol that contains three pieces of information (figure 6.25): Four electrons in a p subshell. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic. From the orbital. What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al.
From denkerensterly.blogspot.com
Predict the Ground State Electron Configuration Co3+ Denker Ensterly What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al Four electrons in a p subshell. The diagram of an electron configuration specifies the subshell (n and l value, with letter symbol) and. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic. From the orbital diagram, we can write the electron configuration in an abbreviated form in which the occupied orbitals. What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al.
From www.sciencefacts.net
Electron Shell Definition & Number of Electrons in Each Shell What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al That is, we follow the three important rules: Four electrons in a p subshell. From the orbital diagram, we can write the electron configuration in an abbreviated form in which the occupied orbitals are identified by their principal quantum number. We describe an electron configuration with a symbol that contains three pieces of information (figure 6.25): The diagram of an. What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al.
From animalia-life.club
Zinc Electron Configuration What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic. The electrons in titanium are arranged in their orbitals as shown. Four electrons in a p subshell. From the orbital diagram, we can write the electron configuration in an abbreviated form in which the occupied orbitals are identified by their principal quantum. What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al.
From ar.inspiredpencil.com
Aluminium Electron Configuration What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al That is, we follow the three important rules: From the orbital diagram, we can write the electron configuration in an abbreviated form in which the occupied orbitals are identified by their principal quantum number. The electrons in titanium are arranged in their orbitals as shown. We describe an electron configuration with a symbol that contains three pieces of information (figure. What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al.
From www.pinterest.com
Why Are Atoms With 8 Valence Electrons So Stable? Covalent bonding What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al The diagram of an electron configuration specifies the subshell (n and l value, with letter symbol) and. We describe an electron configuration with a symbol that contains three pieces of information (figure 6.25): That is, we follow the three important rules: From the orbital diagram, we can write the electron configuration in an abbreviated form in which the occupied orbitals. What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al.
From www.numerade.com
SOLVED The electron configuration of an atom in its ground state is What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al The electrons in titanium are arranged in their orbitals as shown. What is the electron configuration of: From the orbital diagram, we can write the electron configuration in an abbreviated form in which the occupied orbitals are identified by their principal quantum number. Four electrons in a p subshell. We describe an electron configuration with a symbol that contains three. What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al.
From valenceelectrons.com
How to Write the Electron Configuration for Aluminium (Al)? What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al That is, we follow the three important rules: From the orbital diagram, we can write the electron configuration in an abbreviated form in which the occupied orbitals are identified by their principal quantum number. Four electrons in a p subshell. We describe an electron configuration with a symbol that contains three pieces of information (figure 6.25): What is the electron. What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al.
From periodictable.me
How Can We Find Electron Configuration For AL (Aluminium) What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al From the orbital diagram, we can write the electron configuration in an abbreviated form in which the occupied orbitals are identified by their principal quantum number. What is the electron configuration of: The electrons in titanium are arranged in their orbitals as shown. We describe an electron configuration with a symbol that contains three pieces of information (figure 6.25): The. What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al.
From opentextbc.ca
Organization of Electrons in Atoms Introductory Chemistry 1st What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al Four electrons in a p subshell. We describe an electron configuration with a symbol that contains three pieces of information (figure 6.25): What is the electron configuration of: That is, we follow the three important rules: The electrons in titanium are arranged in their orbitals as shown. The diagram of an electron configuration specifies the subshell (n and l value,. What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al.
From stock.adobe.com
Table showing electron orbital configuration of the smallest atoms What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al The diagram of an electron configuration specifies the subshell (n and l value, with letter symbol) and. We describe an electron configuration with a symbol that contains three pieces of information (figure 6.25): That is, we follow the three important rules: This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic. From. What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al.
From chem.libretexts.org
2.2 Electron Configurations Chemistry LibreTexts What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al Four electrons in a p subshell. The diagram of an electron configuration specifies the subshell (n and l value, with letter symbol) and. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic. That is, we follow the three important rules: What is the electron configuration of: We describe an electron configuration. What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al.
From newtondesk.com
Electron Configuration of Elements Chemistry Periodic Table What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al We describe an electron configuration with a symbol that contains three pieces of information (figure 6.25): From the orbital diagram, we can write the electron configuration in an abbreviated form in which the occupied orbitals are identified by their principal quantum number. That is, we follow the three important rules: This electron configuration calculator will instantly show you the distribution. What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al.
From www.newtondesk.com
Aluminium Al (Element 13) of Periodic Table Elements FlashCards What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al Four electrons in a p subshell. From the orbital diagram, we can write the electron configuration in an abbreviated form in which the occupied orbitals are identified by their principal quantum number. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic. What is the electron configuration of: That is, we follow. What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al.
From www.breakingatom.com
Electron Configuration and Structure What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic. The electrons in titanium are arranged in their orbitals as shown. We describe an electron configuration with a symbol that contains three pieces of information (figure 6.25): That is, we follow the three important rules: What is the electron configuration of: From. What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al.
From www.youtube.com
Electron Configuration of Ions YouTube What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al Four electrons in a p subshell. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic. The diagram of an electron configuration specifies the subshell (n and l value, with letter symbol) and. That is, we follow the three important rules: From the orbital diagram, we can write the electron configuration in. What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al.
From www.chegg.com
Solved A neutral atom has the following electron What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al The electrons in titanium are arranged in their orbitals as shown. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic. From the orbital diagram, we can write the electron configuration in an abbreviated form in which the occupied orbitals are identified by their principal quantum number. We describe an electron configuration. What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al.
From homedeso.vercel.app
Group 1 Periodic Table Electron Configuration What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al What is the electron configuration of: From the orbital diagram, we can write the electron configuration in an abbreviated form in which the occupied orbitals are identified by their principal quantum number. Four electrons in a p subshell. The diagram of an electron configuration specifies the subshell (n and l value, with letter symbol) and. We describe an electron configuration. What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al.
From bmp-level.blogspot.com
What Is An Electron Shell Definition bmplevel What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al The diagram of an electron configuration specifies the subshell (n and l value, with letter symbol) and. That is, we follow the three important rules: What is the electron configuration of: We describe an electron configuration with a symbol that contains three pieces of information (figure 6.25): From the orbital diagram, we can write the electron configuration in an abbreviated. What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID1787201 What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al From the orbital diagram, we can write the electron configuration in an abbreviated form in which the occupied orbitals are identified by their principal quantum number. What is the electron configuration of: This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic. The diagram of an electron configuration specifies the subshell (n. What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al.
From www.chegg.com
Solved What is the electron configuration of the Iron(III) What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al Four electrons in a p subshell. The diagram of an electron configuration specifies the subshell (n and l value, with letter symbol) and. We describe an electron configuration with a symbol that contains three pieces of information (figure 6.25): That is, we follow the three important rules: What is the electron configuration of: From the orbital diagram, we can write. What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al.
From www.thesciencehive.co.uk
Electron Structure* — the science sauce What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al What is the electron configuration of: Four electrons in a p subshell. The electrons in titanium are arranged in their orbitals as shown. That is, we follow the three important rules: From the orbital diagram, we can write the electron configuration in an abbreviated form in which the occupied orbitals are identified by their principal quantum number. The diagram of. What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al.
From sciencenotes.org
List of Electron Configurations of Elements What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al What is the electron configuration of: From the orbital diagram, we can write the electron configuration in an abbreviated form in which the occupied orbitals are identified by their principal quantum number. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic. The electrons in titanium are arranged in their orbitals as. What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al.
From aluminumgenjin.blogspot.com
Aluminum Electron Configuration For Aluminum What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic. Four electrons in a p subshell. We describe an electron configuration with a symbol that contains three pieces of information (figure 6.25): The electrons in titanium are arranged in their orbitals as shown. From the orbital diagram, we can write the electron. What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al.
From sciencenotes.org
Electron Shell Diagrams of the 118 Elements What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al From the orbital diagram, we can write the electron configuration in an abbreviated form in which the occupied orbitals are identified by their principal quantum number. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic. Four electrons in a p subshell. The diagram of an electron configuration specifies the subshell (n. What Is The Closed Shell Electron Configuration Of The Following Atoms Ions Al.