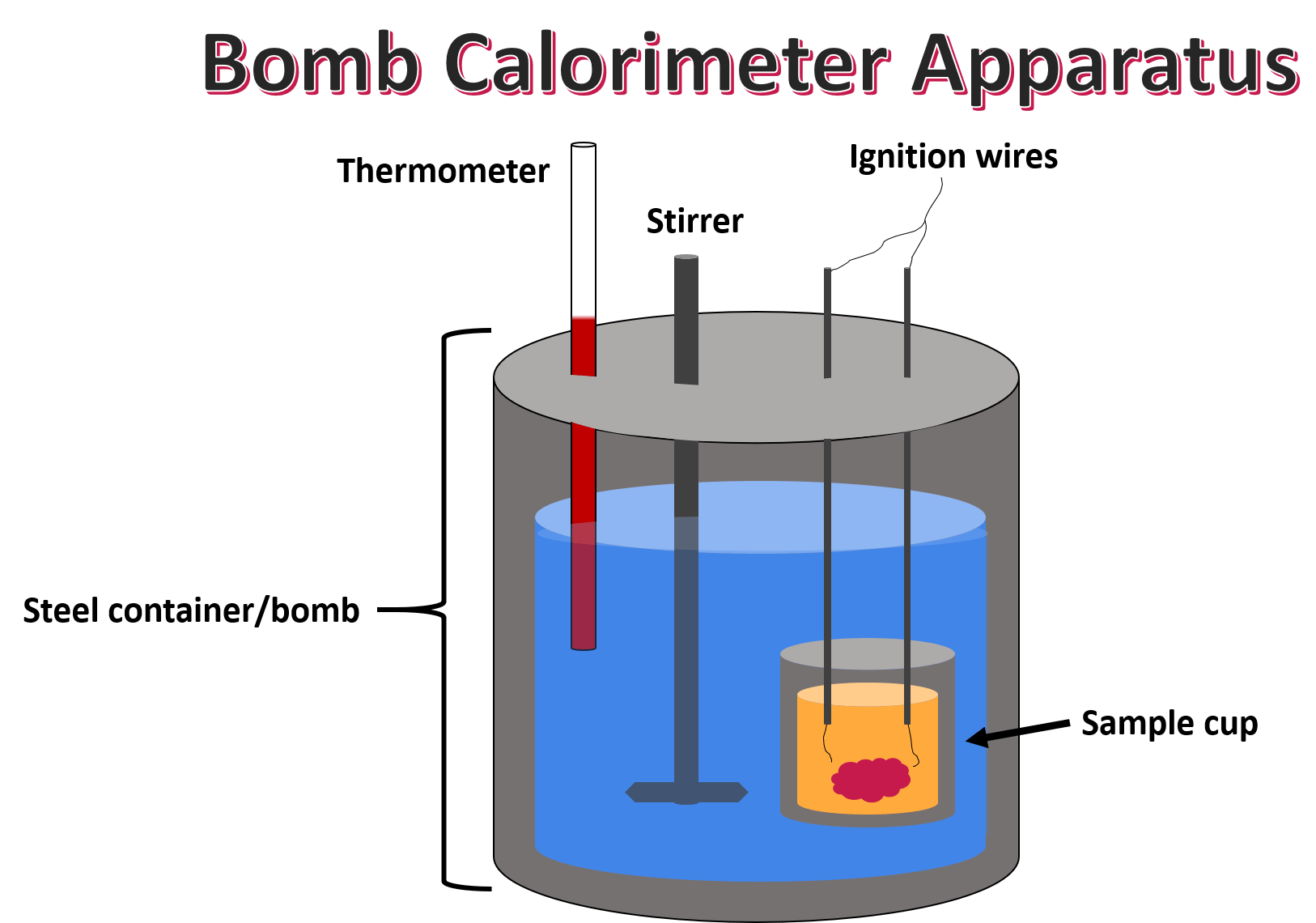
Bomb Calorimeter In Chemistry . constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding. So, for a constant volume, δe = q, and therefore, the bomb calorimeter measures. bomb calorimeter formula : when 0.963 g of benzene, c 6 h 6, is burned in a bomb calorimeter, the temperature of the calorimeter increases by 8.39 °c. Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the calorific value of. The consequence of the calculation is called the amount of combustion, calorification, or btu. bomb calorimetry is generally used to measure the heat of combustion for organic materials. a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. w = p δ v and δ v is zero when the volume is constant. describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. The principle of operation is to saturate the.
from www.expii.com
a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. bomb calorimeter formula : bomb calorimetry is generally used to measure the heat of combustion for organic materials. So, for a constant volume, δe = q, and therefore, the bomb calorimeter measures. w = p δ v and δ v is zero when the volume is constant. The principle of operation is to saturate the. Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the calorific value of. The consequence of the calculation is called the amount of combustion, calorification, or btu. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding. describe a simple calorimeter and explain how it is employed and how its heat capacity is determined.
Bomb Calorimeter — Structure & Function Expii
Bomb Calorimeter In Chemistry bomb calorimetry is generally used to measure the heat of combustion for organic materials. So, for a constant volume, δe = q, and therefore, the bomb calorimeter measures. when 0.963 g of benzene, c 6 h 6, is burned in a bomb calorimeter, the temperature of the calorimeter increases by 8.39 °c. bomb calorimetry is generally used to measure the heat of combustion for organic materials. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding. Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the calorific value of. a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. The principle of operation is to saturate the. bomb calorimeter formula : describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. The consequence of the calculation is called the amount of combustion, calorification, or btu. w = p δ v and δ v is zero when the volume is constant.
From www.vrogue.co
What Is Calorimetry With Pictures vrogue.co Bomb Calorimeter In Chemistry w = p δ v and δ v is zero when the volume is constant. bomb calorimeter formula : So, for a constant volume, δe = q, and therefore, the bomb calorimeter measures. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding. The consequence of the calculation. Bomb Calorimeter In Chemistry.
From saylordotorg.github.io
Calorimetry Bomb Calorimeter In Chemistry Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the calorific value of. w = p δ v and δ v is zero when the volume is constant. The principle of operation is to saturate the. bomb calorimetry is generally used to measure the heat of combustion for organic. Bomb Calorimeter In Chemistry.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii Bomb Calorimeter In Chemistry w = p δ v and δ v is zero when the volume is constant. describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. bomb calorimeter formula : when 0.963 g of benzene, c 6 h 6, is burned in a bomb calorimeter, the temperature of the calorimeter. Bomb Calorimeter In Chemistry.
From commons.trincoll.edu
Parr 1455 Bomb Calorimeter Trinity College Chemistry Department Bomb Calorimeter In Chemistry Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the calorific value of. So, for a constant volume, δe = q, and therefore, the bomb calorimeter measures. when 0.963 g of benzene, c 6 h 6, is burned in a bomb calorimeter, the temperature of the calorimeter increases by 8.39. Bomb Calorimeter In Chemistry.
From courses.lumenlearning.com
Calorimetry Chemistry Bomb Calorimeter In Chemistry when 0.963 g of benzene, c 6 h 6, is burned in a bomb calorimeter, the temperature of the calorimeter increases by 8.39 °c. a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. w = p δ. Bomb Calorimeter In Chemistry.
From www.youtube.com
Bomb Calorimetry Introduction Physical Chemistry Laboratory YouTube Bomb Calorimeter In Chemistry a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. So, for a constant volume, δe = q, and therefore, the bomb calorimeter measures. The principle of operation is to saturate the. bomb calorimetry is generally used to measure. Bomb Calorimeter In Chemistry.
From studylib.net
Bomb Calorimeter Bomb Calorimeter In Chemistry a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. w = p δ v and δ v is zero when. Bomb Calorimeter In Chemistry.
From www.vrogue.co
What Is Calorimetry With Pictures vrogue.co Bomb Calorimeter In Chemistry The principle of operation is to saturate the. bomb calorimetry is generally used to measure the heat of combustion for organic materials. The consequence of the calculation is called the amount of combustion, calorification, or btu. a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in. Bomb Calorimeter In Chemistry.
From chemlab.truman.edu
Parr 1341 Bomb Calorimeter Chem Lab Bomb Calorimeter In Chemistry describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the calorific value of. bomb calorimetry is generally used to measure the heat of combustion for organic materials. bomb calorimeter formula :. Bomb Calorimeter In Chemistry.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Bomb Calorimeter In Chemistry w = p δ v and δ v is zero when the volume is constant. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding. The consequence of the calculation is called the amount of combustion, calorification, or btu. when 0.963 g of benzene, c 6 h 6,. Bomb Calorimeter In Chemistry.
From chem.libretexts.org
11.5 Reaction Calorimetry Chemistry LibreTexts Bomb Calorimeter In Chemistry So, for a constant volume, δe = q, and therefore, the bomb calorimeter measures. when 0.963 g of benzene, c 6 h 6, is burned in a bomb calorimeter, the temperature of the calorimeter increases by 8.39 °c. The consequence of the calculation is called the amount of combustion, calorification, or btu. a bomb calorimeter is used to. Bomb Calorimeter In Chemistry.
From www.studypool.com
SOLUTION Bomb calorimeter explain with diagram and example? Studypool Bomb Calorimeter In Chemistry bomb calorimetry is generally used to measure the heat of combustion for organic materials. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding. The principle of operation is to saturate the. The consequence of the calculation is called the amount of combustion, calorification, or btu. when 0.963. Bomb Calorimeter In Chemistry.
From www.britannica.com
Bomb calorimeter measurement device Britannica Bomb Calorimeter In Chemistry constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding. bomb calorimeter formula : The consequence of the calculation is called the amount of combustion, calorification, or btu. when 0.963 g of benzene, c 6 h 6, is burned in a bomb calorimeter, the temperature of the calorimeter. Bomb Calorimeter In Chemistry.
From www.thoughtco.com
Calorimeter Definition in Chemistry Bomb Calorimeter In Chemistry a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. when 0.963 g of benzene, c 6 h 6, is burned in a bomb calorimeter, the temperature of the calorimeter increases by 8.39 °c. w = p δ. Bomb Calorimeter In Chemistry.
From www.slideserve.com
PPT TOPIC 8 THERMOCHEMISTRY PowerPoint Presentation, free download ID3087554 Bomb Calorimeter In Chemistry The principle of operation is to saturate the. The consequence of the calculation is called the amount of combustion, calorification, or btu. w = p δ v and δ v is zero when the volume is constant. bomb calorimetry is generally used to measure the heat of combustion for organic materials. when 0.963 g of benzene, c. Bomb Calorimeter In Chemistry.
From www.slideserve.com
PPT Bomb Calorimetry PowerPoint Presentation ID3206969 Bomb Calorimeter In Chemistry constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding. when 0.963 g of benzene, c 6 h 6, is burned in a bomb calorimeter, the temperature of the calorimeter increases by 8.39 °c. w = p δ v and δ v is zero when the volume is. Bomb Calorimeter In Chemistry.
From www.fire-testing.com
Oxygen Bomb Calorimeter Fire Testing Technology Bomb Calorimeter In Chemistry So, for a constant volume, δe = q, and therefore, the bomb calorimeter measures. Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the calorific value of. w = p δ v and δ v is zero when the volume is constant. constant volume calorimetry, also know as bomb. Bomb Calorimeter In Chemistry.
From holycrackers.info
IKA BOMB CALORIMETER PDF Bomb Calorimeter In Chemistry bomb calorimeter formula : a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding. bomb calorimetry is generally. Bomb Calorimeter In Chemistry.
From www.youtube.com
Bomb Calorimetry Problem (Chemistry) YouTube Bomb Calorimeter In Chemistry The principle of operation is to saturate the. a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. w = p. Bomb Calorimeter In Chemistry.
From www.slideserve.com
PPT Chemistry A Molecular Approach , 1 st Edition Nivaldo Tro PowerPoint Presentation ID Bomb Calorimeter In Chemistry constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding. bomb calorimetry is generally used to measure the heat of combustion for organic materials. w = p δ v and δ v is zero when the volume is constant. So, for a constant volume, δe = q, and. Bomb Calorimeter In Chemistry.
From martinfersbanks.blogspot.com
Is a Bomb Calorimeter Constant Pressure Bomb Calorimeter In Chemistry The consequence of the calculation is called the amount of combustion, calorification, or btu. a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. The principle of operation is to saturate the. So, for a constant volume, δe = q,. Bomb Calorimeter In Chemistry.
From exosbbnfj.blob.core.windows.net
Calorimetry Ap Chemistry at Michael Faust blog Bomb Calorimeter In Chemistry Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the calorific value of. bomb calorimetry is generally used to measure the heat of combustion for organic materials. when 0.963 g of benzene, c 6 h 6, is burned in a bomb calorimeter, the temperature of the calorimeter increases by. Bomb Calorimeter In Chemistry.
From animalia-life.club
Simple Bomb Calorimeter Bomb Calorimeter In Chemistry constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding. Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the calorific value of. bomb calorimetry is generally used to measure the heat of combustion for organic materials. a bomb. Bomb Calorimeter In Chemistry.
From martinfersbanks.blogspot.com
Is a Bomb Calorimeter Constant Pressure Bomb Calorimeter In Chemistry w = p δ v and δ v is zero when the volume is constant. The consequence of the calculation is called the amount of combustion, calorification, or btu. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding. describe a simple calorimeter and explain how it is. Bomb Calorimeter In Chemistry.
From exobdylqi.blob.core.windows.net
Bomb Calorimeter What Does It Do at Joann Guarino blog Bomb Calorimeter In Chemistry describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. So, for a constant volume, δe = q, and therefore, the bomb calorimeter measures. The principle of operation is to saturate the. w = p δ v and δ v is zero when the volume is constant. Dulong’s formula used to. Bomb Calorimeter In Chemistry.
From www.youtube.com
Physical Chemistry iBook Bomb Calorimetry YouTube Bomb Calorimeter In Chemistry bomb calorimetry is generally used to measure the heat of combustion for organic materials. Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the calorific value of. The principle of operation is to saturate the. when 0.963 g of benzene, c 6 h 6, is burned in a bomb. Bomb Calorimeter In Chemistry.
From studylib.net
1b bomb calorimeter Bomb Calorimeter In Chemistry The consequence of the calculation is called the amount of combustion, calorification, or btu. describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the calorific value of. when 0.963 g of benzene,. Bomb Calorimeter In Chemistry.
From www.slideserve.com
PPT Chapter 6 Thermochemistry PowerPoint Presentation, free download ID5118958 Bomb Calorimeter In Chemistry The consequence of the calculation is called the amount of combustion, calorification, or btu. So, for a constant volume, δe = q, and therefore, the bomb calorimeter measures. a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. Dulong’s formula. Bomb Calorimeter In Chemistry.
From www.pinterest.com
Calorimetry Bomb Calorimeter Experiment Science fair, Homeschool and Chemistry Bomb Calorimeter In Chemistry Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the calorific value of. a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. when 0.963 g of benzene, c 6. Bomb Calorimeter In Chemistry.
From www.youtube.com
Bomb Calorimetry YouTube Bomb Calorimeter In Chemistry when 0.963 g of benzene, c 6 h 6, is burned in a bomb calorimeter, the temperature of the calorimeter increases by 8.39 °c. Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the calorific value of. constant volume calorimetry, also know as bomb calorimetry, is used to measure. Bomb Calorimeter In Chemistry.
From www.youtube.com
Bomb calorimeter construction of bomb calorimeter working of bomb calorimeter b.tech Bomb Calorimeter In Chemistry a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding. So, for a constant volume, δe = q, and therefore,. Bomb Calorimeter In Chemistry.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Bomb Calorimeter In Chemistry a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. w = p δ v and δ v is zero when the volume is constant. bomb calorimeter formula : bomb calorimetry is generally used to measure the. Bomb Calorimeter In Chemistry.
From www.studypool.com
SOLUTION Bomb calorimeter explain with diagram and example? Studypool Bomb Calorimeter In Chemistry So, for a constant volume, δe = q, and therefore, the bomb calorimeter measures. w = p δ v and δ v is zero when the volume is constant. describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. Dulong’s formula used to calculate the theoretical calorific value of fuel if. Bomb Calorimeter In Chemistry.
From www.youtube.com
Bomb calorimeter class 11 chemistry Ch7 Lecture10B Chemistry ideas YouTube Bomb Calorimeter In Chemistry The consequence of the calculation is called the amount of combustion, calorification, or btu. bomb calorimetry is generally used to measure the heat of combustion for organic materials. describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. when 0.963 g of benzene, c 6 h 6, is burned in. Bomb Calorimeter In Chemistry.
From www.youtube.com
VCE Chemistry Unit 4 Bomb Calorimeter Example YouTube Bomb Calorimeter In Chemistry The principle of operation is to saturate the. bomb calorimeter formula : a bomb calorimeter is used to measure, under controlled conditions, the heat emitted by a sample burned under an oxygen atmosphere in a closed vessel (bomb) surrounded by water. Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available. Bomb Calorimeter In Chemistry.