Product Quality Risk Assessment . risk assessment of product quality attributes linked to control strategy. quality risk management is a systematic process for the assessment, control, communication and review of risks to the quality of the drug (medicinal) product across. ich q9 maps out a systematic approach to quality risk management (qrm) throughout the lifecycle of your pharmaceutical product. The process is composed of. the guidance is a targeted revision of the 2006 guidance for industry “q9 quality risk management” and addresses. this document provides principles and examples of tools for quality risk management that can be applied to different. Proactive monitoring & trending of.
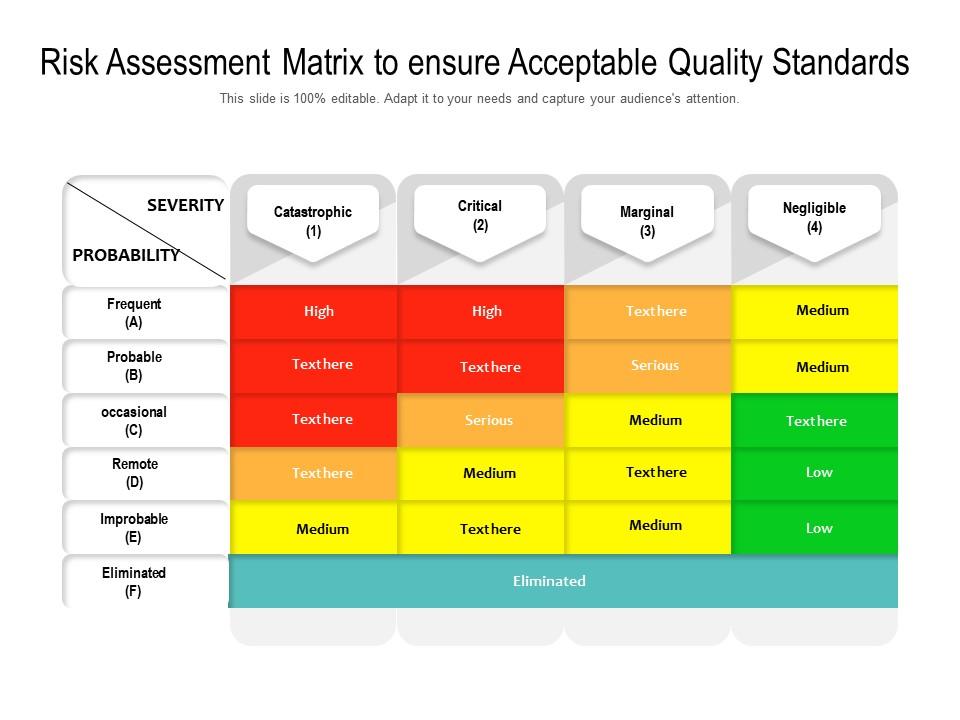
from mungfali.com
quality risk management is a systematic process for the assessment, control, communication and review of risks to the quality of the drug (medicinal) product across. risk assessment of product quality attributes linked to control strategy. Proactive monitoring & trending of. this document provides principles and examples of tools for quality risk management that can be applied to different. the guidance is a targeted revision of the 2006 guidance for industry “q9 quality risk management” and addresses. ich q9 maps out a systematic approach to quality risk management (qrm) throughout the lifecycle of your pharmaceutical product. The process is composed of.
Quality Risk Matrix
Product Quality Risk Assessment quality risk management is a systematic process for the assessment, control, communication and review of risks to the quality of the drug (medicinal) product across. this document provides principles and examples of tools for quality risk management that can be applied to different. The process is composed of. ich q9 maps out a systematic approach to quality risk management (qrm) throughout the lifecycle of your pharmaceutical product. risk assessment of product quality attributes linked to control strategy. Proactive monitoring & trending of. the guidance is a targeted revision of the 2006 guidance for industry “q9 quality risk management” and addresses. quality risk management is a systematic process for the assessment, control, communication and review of risks to the quality of the drug (medicinal) product across.
From www.americanpharmaceuticalreview.com
Critical Quality Attributes Assessment and Testing Strategy for Biotherapeutics Development Product Quality Risk Assessment The process is composed of. the guidance is a targeted revision of the 2006 guidance for industry “q9 quality risk management” and addresses. risk assessment of product quality attributes linked to control strategy. this document provides principles and examples of tools for quality risk management that can be applied to different. quality risk management is a. Product Quality Risk Assessment.
From www.getreskilled.com
Quality Risk Management in the Pharma View 8 QRM Tools Product Quality Risk Assessment ich q9 maps out a systematic approach to quality risk management (qrm) throughout the lifecycle of your pharmaceutical product. quality risk management is a systematic process for the assessment, control, communication and review of risks to the quality of the drug (medicinal) product across. Proactive monitoring & trending of. this document provides principles and examples of tools. Product Quality Risk Assessment.
From www.slideteam.net
Scope And Principles Of Quality Risk Assessment QRM Ppt Infographics PPT Example Product Quality Risk Assessment risk assessment of product quality attributes linked to control strategy. quality risk management is a systematic process for the assessment, control, communication and review of risks to the quality of the drug (medicinal) product across. the guidance is a targeted revision of the 2006 guidance for industry “q9 quality risk management” and addresses. The process is composed. Product Quality Risk Assessment.
From e1f96b2476b8058be005-f86fb06f9ff65ea438446e070ce5ac31.ssl.cf2.rackcdn.com
Table 9.07 Quality Risk Assessment Report Example Product Quality Risk Assessment this document provides principles and examples of tools for quality risk management that can be applied to different. ich q9 maps out a systematic approach to quality risk management (qrm) throughout the lifecycle of your pharmaceutical product. the guidance is a targeted revision of the 2006 guidance for industry “q9 quality risk management” and addresses. quality. Product Quality Risk Assessment.
From insideofpharma.blogspot.com
Pharma Knowledge Quality risk management for new product introduction Product Quality Risk Assessment risk assessment of product quality attributes linked to control strategy. this document provides principles and examples of tools for quality risk management that can be applied to different. the guidance is a targeted revision of the 2006 guidance for industry “q9 quality risk management” and addresses. The process is composed of. quality risk management is a. Product Quality Risk Assessment.
From www.slidegeeks.com
Risk Ranking And Filtering Method Quality Risk Assessment Designs PDF Product Quality Risk Assessment risk assessment of product quality attributes linked to control strategy. this document provides principles and examples of tools for quality risk management that can be applied to different. the guidance is a targeted revision of the 2006 guidance for industry “q9 quality risk management” and addresses. ich q9 maps out a systematic approach to quality risk. Product Quality Risk Assessment.
From www.americanpharmaceuticalreview.com
Critical Quality Attributes Assessment and Testing Strategy for Biotherapeutics Development Product Quality Risk Assessment the guidance is a targeted revision of the 2006 guidance for industry “q9 quality risk management” and addresses. ich q9 maps out a systematic approach to quality risk management (qrm) throughout the lifecycle of your pharmaceutical product. Proactive monitoring & trending of. this document provides principles and examples of tools for quality risk management that can be. Product Quality Risk Assessment.
From www.scribd.com
Qualitative Risk Analysis Matrix Level of Risk Product Quality Risk Assessment ich q9 maps out a systematic approach to quality risk management (qrm) throughout the lifecycle of your pharmaceutical product. this document provides principles and examples of tools for quality risk management that can be applied to different. The process is composed of. risk assessment of product quality attributes linked to control strategy. quality risk management is. Product Quality Risk Assessment.
From pharmabeginers.com
SOP for Quality Risk Management (Guideline ICH Q9) Pharma Beginners Product Quality Risk Assessment this document provides principles and examples of tools for quality risk management that can be applied to different. quality risk management is a systematic process for the assessment, control, communication and review of risks to the quality of the drug (medicinal) product across. The process is composed of. the guidance is a targeted revision of the 2006. Product Quality Risk Assessment.
From www.scribd.com
Product Quality Risk Assessment Verification And Validation Safety Product Quality Risk Assessment quality risk management is a systematic process for the assessment, control, communication and review of risks to the quality of the drug (medicinal) product across. The process is composed of. the guidance is a targeted revision of the 2006 guidance for industry “q9 quality risk management” and addresses. Proactive monitoring & trending of. this document provides principles. Product Quality Risk Assessment.
From www.riskpal.com
Risk Assessment Matrices Tools to Visualise Risk Product Quality Risk Assessment this document provides principles and examples of tools for quality risk management that can be applied to different. Proactive monitoring & trending of. The process is composed of. ich q9 maps out a systematic approach to quality risk management (qrm) throughout the lifecycle of your pharmaceutical product. the guidance is a targeted revision of the 2006 guidance. Product Quality Risk Assessment.
From www.slideteam.net
Overview Of A Typical Quality Risk Management Process Quality Risk Management Presentation Product Quality Risk Assessment Proactive monitoring & trending of. the guidance is a targeted revision of the 2006 guidance for industry “q9 quality risk management” and addresses. risk assessment of product quality attributes linked to control strategy. The process is composed of. ich q9 maps out a systematic approach to quality risk management (qrm) throughout the lifecycle of your pharmaceutical product.. Product Quality Risk Assessment.
From www.slideserve.com
PPT Product Development Case Study Overview PowerPoint Presentation ID524319 Product Quality Risk Assessment The process is composed of. ich q9 maps out a systematic approach to quality risk management (qrm) throughout the lifecycle of your pharmaceutical product. this document provides principles and examples of tools for quality risk management that can be applied to different. risk assessment of product quality attributes linked to control strategy. quality risk management is. Product Quality Risk Assessment.
From qualityinspection.org
How To Do A Product Risk Assessment For Safety? Product Quality Risk Assessment Proactive monitoring & trending of. risk assessment of product quality attributes linked to control strategy. the guidance is a targeted revision of the 2006 guidance for industry “q9 quality risk management” and addresses. ich q9 maps out a systematic approach to quality risk management (qrm) throughout the lifecycle of your pharmaceutical product. this document provides principles. Product Quality Risk Assessment.
From www.slideteam.net
Risk Assessment Of Critical Quality Attributes Pharmaceutical Development New Medicine Product Quality Risk Assessment ich q9 maps out a systematic approach to quality risk management (qrm) throughout the lifecycle of your pharmaceutical product. The process is composed of. the guidance is a targeted revision of the 2006 guidance for industry “q9 quality risk management” and addresses. Proactive monitoring & trending of. this document provides principles and examples of tools for quality. Product Quality Risk Assessment.
From www.pharmaceuticalonline.com
Quality Risk Management 101 QRM And The Product Life Cycle Product Quality Risk Assessment The process is composed of. quality risk management is a systematic process for the assessment, control, communication and review of risks to the quality of the drug (medicinal) product across. the guidance is a targeted revision of the 2006 guidance for industry “q9 quality risk management” and addresses. this document provides principles and examples of tools for. Product Quality Risk Assessment.
From www.slideteam.net
Top 7 Risk Management Chart Templates with Examples and Samples Product Quality Risk Assessment quality risk management is a systematic process for the assessment, control, communication and review of risks to the quality of the drug (medicinal) product across. Proactive monitoring & trending of. the guidance is a targeted revision of the 2006 guidance for industry “q9 quality risk management” and addresses. risk assessment of product quality attributes linked to control. Product Quality Risk Assessment.
From cqeacademy.com
Quality Risk Management Tools Product Quality Risk Assessment ich q9 maps out a systematic approach to quality risk management (qrm) throughout the lifecycle of your pharmaceutical product. quality risk management is a systematic process for the assessment, control, communication and review of risks to the quality of the drug (medicinal) product across. Proactive monitoring & trending of. this document provides principles and examples of tools. Product Quality Risk Assessment.
From www.presentationeze.com
Supplier Risk Assessment PresentationEZE Product Quality Risk Assessment risk assessment of product quality attributes linked to control strategy. The process is composed of. the guidance is a targeted revision of the 2006 guidance for industry “q9 quality risk management” and addresses. this document provides principles and examples of tools for quality risk management that can be applied to different. ich q9 maps out a. Product Quality Risk Assessment.
From www.semanticscholar.org
Quality Risk Management Principles and Industry Case Studies Semantic Scholar Product Quality Risk Assessment risk assessment of product quality attributes linked to control strategy. the guidance is a targeted revision of the 2006 guidance for industry “q9 quality risk management” and addresses. this document provides principles and examples of tools for quality risk management that can be applied to different. Proactive monitoring & trending of. ich q9 maps out a. Product Quality Risk Assessment.
From cqeacademy.com
Product & Process Design for the CQE Product Quality Risk Assessment this document provides principles and examples of tools for quality risk management that can be applied to different. The process is composed of. the guidance is a targeted revision of the 2006 guidance for industry “q9 quality risk management” and addresses. quality risk management is a systematic process for the assessment, control, communication and review of risks. Product Quality Risk Assessment.
From pharmabeginers.com
SOP for Quality Risk Management (Guideline ICH Q9) Pharma Beginners Product Quality Risk Assessment The process is composed of. ich q9 maps out a systematic approach to quality risk management (qrm) throughout the lifecycle of your pharmaceutical product. this document provides principles and examples of tools for quality risk management that can be applied to different. quality risk management is a systematic process for the assessment, control, communication and review of. Product Quality Risk Assessment.
From www.slidegeeks.com
Product Quality Risk Assessment Strategy With System Analysis Ppt PowerPoint Presentation File Product Quality Risk Assessment Proactive monitoring & trending of. ich q9 maps out a systematic approach to quality risk management (qrm) throughout the lifecycle of your pharmaceutical product. this document provides principles and examples of tools for quality risk management that can be applied to different. risk assessment of product quality attributes linked to control strategy. quality risk management is. Product Quality Risk Assessment.
From www.kevinian.com
How to Complete a Risk Assessment Kevin Ian Schmidt Product Quality Risk Assessment this document provides principles and examples of tools for quality risk management that can be applied to different. The process is composed of. risk assessment of product quality attributes linked to control strategy. the guidance is a targeted revision of the 2006 guidance for industry “q9 quality risk management” and addresses. ich q9 maps out a. Product Quality Risk Assessment.
From www.slideteam.net
Quality Assessment Scope And Principles Of Quality Risk Assessment PPT Presentation Product Quality Risk Assessment the guidance is a targeted revision of the 2006 guidance for industry “q9 quality risk management” and addresses. risk assessment of product quality attributes linked to control strategy. quality risk management is a systematic process for the assessment, control, communication and review of risks to the quality of the drug (medicinal) product across. this document provides. Product Quality Risk Assessment.
From www.slideteam.net
Initial Risk Assessment Of Critical Quality Attributes Quality By Design For Generic Drugs Product Quality Risk Assessment quality risk management is a systematic process for the assessment, control, communication and review of risks to the quality of the drug (medicinal) product across. risk assessment of product quality attributes linked to control strategy. Proactive monitoring & trending of. this document provides principles and examples of tools for quality risk management that can be applied to. Product Quality Risk Assessment.
From www.slideteam.net
Product Quality Risk Management Plan Flow Chart Presentation Graphics Presentation Product Quality Risk Assessment ich q9 maps out a systematic approach to quality risk management (qrm) throughout the lifecycle of your pharmaceutical product. the guidance is a targeted revision of the 2006 guidance for industry “q9 quality risk management” and addresses. this document provides principles and examples of tools for quality risk management that can be applied to different. Proactive monitoring. Product Quality Risk Assessment.
From www.slideteam.net
Overview Of A Typical Quality Risk Management Process QRM Product Quality Risk Assessment Proactive monitoring & trending of. ich q9 maps out a systematic approach to quality risk management (qrm) throughout the lifecycle of your pharmaceutical product. The process is composed of. this document provides principles and examples of tools for quality risk management that can be applied to different. risk assessment of product quality attributes linked to control strategy.. Product Quality Risk Assessment.
From ispe.org
Quality Risk Management for Legacy Products in CMOs ISPE International Society for Product Quality Risk Assessment the guidance is a targeted revision of the 2006 guidance for industry “q9 quality risk management” and addresses. ich q9 maps out a systematic approach to quality risk management (qrm) throughout the lifecycle of your pharmaceutical product. Proactive monitoring & trending of. risk assessment of product quality attributes linked to control strategy. The process is composed of.. Product Quality Risk Assessment.
From www.slidegeeks.com
Overview Of A Typical Quality Risk Management Process Quality Risk Assessment Elements PDF Product Quality Risk Assessment ich q9 maps out a systematic approach to quality risk management (qrm) throughout the lifecycle of your pharmaceutical product. quality risk management is a systematic process for the assessment, control, communication and review of risks to the quality of the drug (medicinal) product across. risk assessment of product quality attributes linked to control strategy. The process is. Product Quality Risk Assessment.
From www.researchgate.net
Scheme for quality risk management. Download Scientific Diagram Product Quality Risk Assessment risk assessment of product quality attributes linked to control strategy. the guidance is a targeted revision of the 2006 guidance for industry “q9 quality risk management” and addresses. this document provides principles and examples of tools for quality risk management that can be applied to different. ich q9 maps out a systematic approach to quality risk. Product Quality Risk Assessment.
From blog.qatestlab.com
How to Identify and Manage Testing Risks? QATestLab Blog Product Quality Risk Assessment quality risk management is a systematic process for the assessment, control, communication and review of risks to the quality of the drug (medicinal) product across. Proactive monitoring & trending of. this document provides principles and examples of tools for quality risk management that can be applied to different. risk assessment of product quality attributes linked to control. Product Quality Risk Assessment.
From www.slideteam.net
Scope And Principles Of Quality Risk Assessment Quality Risk Management Presentation Graphics Product Quality Risk Assessment ich q9 maps out a systematic approach to quality risk management (qrm) throughout the lifecycle of your pharmaceutical product. The process is composed of. the guidance is a targeted revision of the 2006 guidance for industry “q9 quality risk management” and addresses. quality risk management is a systematic process for the assessment, control, communication and review of. Product Quality Risk Assessment.
From www.sampletemplates.com
FREE 7+ Sample Product Risk Assessment Templates in PDF MS Word Product Quality Risk Assessment ich q9 maps out a systematic approach to quality risk management (qrm) throughout the lifecycle of your pharmaceutical product. quality risk management is a systematic process for the assessment, control, communication and review of risks to the quality of the drug (medicinal) product across. this document provides principles and examples of tools for quality risk management that. Product Quality Risk Assessment.
From mungfali.com
Quality Risk Matrix Product Quality Risk Assessment Proactive monitoring & trending of. ich q9 maps out a systematic approach to quality risk management (qrm) throughout the lifecycle of your pharmaceutical product. quality risk management is a systematic process for the assessment, control, communication and review of risks to the quality of the drug (medicinal) product across. The process is composed of. risk assessment of. Product Quality Risk Assessment.