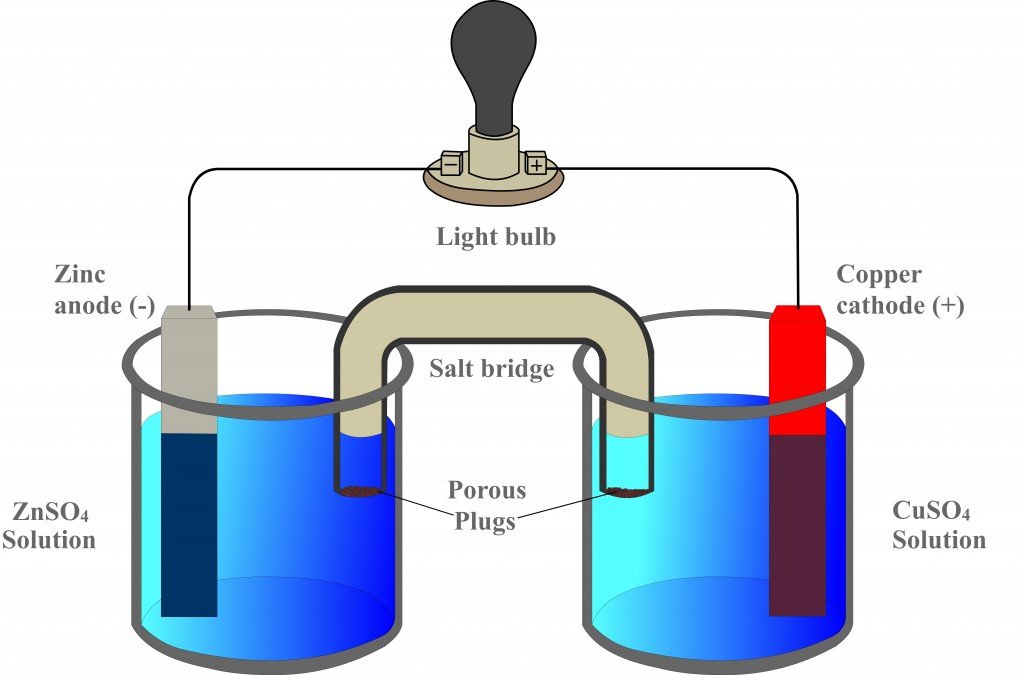
The Galvanic Cell Worksheet . in this activity, students will use a simulation to create a variety of galvanic/voltaic cells with different. However, one is galvanic and one is electrolytic. Make you indicate all of. Cations always flow toward the anode. a galvanic cell may have a negative or positive cell potential. As the reaction in this cell takes place, the. The cells in problems 6 and 7 have almost the same components. a galvanic cell or voltaic cell is a device in which a redox reaction, such as the one in equation (4), spontaneously occurs and. this worksheet will cover galvanic cells and their components, such as anodes, cathodes, wires, salt bridges, and electrolyte. 6.the overall reaction in a electrochemical cell is zn(s) + cu2+(aq) ® zn2+(aq) + cu(s). draw (graphically) a galvanic cell that takes advantage of the spontaneous redox reaction indicated in q2. calculate the standard cell potential produced by a galvanic cell consisting of.
from www.scienceabc.com
The cells in problems 6 and 7 have almost the same components. calculate the standard cell potential produced by a galvanic cell consisting of. this worksheet will cover galvanic cells and their components, such as anodes, cathodes, wires, salt bridges, and electrolyte. As the reaction in this cell takes place, the. However, one is galvanic and one is electrolytic. in this activity, students will use a simulation to create a variety of galvanic/voltaic cells with different. a galvanic cell or voltaic cell is a device in which a redox reaction, such as the one in equation (4), spontaneously occurs and. Make you indicate all of. a galvanic cell may have a negative or positive cell potential. 6.the overall reaction in a electrochemical cell is zn(s) + cu2+(aq) ® zn2+(aq) + cu(s).
Galvanic Cell Definition, Diagram And Working
The Galvanic Cell Worksheet draw (graphically) a galvanic cell that takes advantage of the spontaneous redox reaction indicated in q2. 6.the overall reaction in a electrochemical cell is zn(s) + cu2+(aq) ® zn2+(aq) + cu(s). in this activity, students will use a simulation to create a variety of galvanic/voltaic cells with different. a galvanic cell may have a negative or positive cell potential. calculate the standard cell potential produced by a galvanic cell consisting of. a galvanic cell or voltaic cell is a device in which a redox reaction, such as the one in equation (4), spontaneously occurs and. draw (graphically) a galvanic cell that takes advantage of the spontaneous redox reaction indicated in q2. The cells in problems 6 and 7 have almost the same components. Cations always flow toward the anode. Make you indicate all of. As the reaction in this cell takes place, the. this worksheet will cover galvanic cells and their components, such as anodes, cathodes, wires, salt bridges, and electrolyte. However, one is galvanic and one is electrolytic.
From issuu.com
Electrochemical Cell Worksheet by Olivia Hunter Issuu The Galvanic Cell Worksheet The cells in problems 6 and 7 have almost the same components. this worksheet will cover galvanic cells and their components, such as anodes, cathodes, wires, salt bridges, and electrolyte. draw (graphically) a galvanic cell that takes advantage of the spontaneous redox reaction indicated in q2. a galvanic cell or voltaic cell is a device in which. The Galvanic Cell Worksheet.
From www.studocu.com
Chem 185 Galvanic Cells Prelab GALVANIC CELLS Prelab Reading Read GCN pages 43. Prelab The Galvanic Cell Worksheet Make you indicate all of. a galvanic cell may have a negative or positive cell potential. The cells in problems 6 and 7 have almost the same components. Cations always flow toward the anode. 6.the overall reaction in a electrochemical cell is zn(s) + cu2+(aq) ® zn2+(aq) + cu(s). As the reaction in this cell takes place, the.. The Galvanic Cell Worksheet.
From www.liveworksheets.com
Galvanic 1 worksheet The Galvanic Cell Worksheet a galvanic cell may have a negative or positive cell potential. in this activity, students will use a simulation to create a variety of galvanic/voltaic cells with different. 6.the overall reaction in a electrochemical cell is zn(s) + cu2+(aq) ® zn2+(aq) + cu(s). The cells in problems 6 and 7 have almost the same components. this. The Galvanic Cell Worksheet.
From courses.lumenlearning.com
Galvanic Cells Chemistry The Galvanic Cell Worksheet this worksheet will cover galvanic cells and their components, such as anodes, cathodes, wires, salt bridges, and electrolyte. in this activity, students will use a simulation to create a variety of galvanic/voltaic cells with different. a galvanic cell may have a negative or positive cell potential. a galvanic cell or voltaic cell is a device in. The Galvanic Cell Worksheet.
From www.studypool.com
SOLUTION G9 galvanic cell worksheet Studypool The Galvanic Cell Worksheet The cells in problems 6 and 7 have almost the same components. calculate the standard cell potential produced by a galvanic cell consisting of. However, one is galvanic and one is electrolytic. Make you indicate all of. Cations always flow toward the anode. a galvanic cell may have a negative or positive cell potential. 6.the overall reaction. The Galvanic Cell Worksheet.
From www.studypool.com
SOLUTION Fridayworksheetgalvanic1soln 1 Studypool The Galvanic Cell Worksheet calculate the standard cell potential produced by a galvanic cell consisting of. As the reaction in this cell takes place, the. Make you indicate all of. The cells in problems 6 and 7 have almost the same components. a galvanic cell may have a negative or positive cell potential. Cations always flow toward the anode. draw (graphically). The Galvanic Cell Worksheet.
From ar.inspiredpencil.com
Galvanic Cell Labeled The Galvanic Cell Worksheet this worksheet will cover galvanic cells and their components, such as anodes, cathodes, wires, salt bridges, and electrolyte. The cells in problems 6 and 7 have almost the same components. As the reaction in this cell takes place, the. draw (graphically) a galvanic cell that takes advantage of the spontaneous redox reaction indicated in q2. Make you indicate. The Galvanic Cell Worksheet.
From www.studypool.com
SOLUTION Electrochemistry Worksheet Galvanic Cell 89 grade Studypool The Galvanic Cell Worksheet this worksheet will cover galvanic cells and their components, such as anodes, cathodes, wires, salt bridges, and electrolyte. calculate the standard cell potential produced by a galvanic cell consisting of. As the reaction in this cell takes place, the. draw (graphically) a galvanic cell that takes advantage of the spontaneous redox reaction indicated in q2. Cations always. The Galvanic Cell Worksheet.
From studylib.net
Redox and Galvanic cells The Galvanic Cell Worksheet calculate the standard cell potential produced by a galvanic cell consisting of. Make you indicate all of. 6.the overall reaction in a electrochemical cell is zn(s) + cu2+(aq) ® zn2+(aq) + cu(s). As the reaction in this cell takes place, the. Cations always flow toward the anode. in this activity, students will use a simulation to create. The Galvanic Cell Worksheet.
From www.studypool.com
SOLUTION Galvanic cells exercises Studypool The Galvanic Cell Worksheet calculate the standard cell potential produced by a galvanic cell consisting of. As the reaction in this cell takes place, the. Cations always flow toward the anode. Make you indicate all of. a galvanic cell may have a negative or positive cell potential. However, one is galvanic and one is electrolytic. draw (graphically) a galvanic cell that. The Galvanic Cell Worksheet.
From studylib.net
Information Galvanic Cell Diagram The Galvanic Cell Worksheet Cations always flow toward the anode. However, one is galvanic and one is electrolytic. 6.the overall reaction in a electrochemical cell is zn(s) + cu2+(aq) ® zn2+(aq) + cu(s). calculate the standard cell potential produced by a galvanic cell consisting of. in this activity, students will use a simulation to create a variety of galvanic/voltaic cells with. The Galvanic Cell Worksheet.
From www.nagwa.com
Question Video Selecting the Correct Labels for a Galvanic Cell Nagwa The Galvanic Cell Worksheet calculate the standard cell potential produced by a galvanic cell consisting of. As the reaction in this cell takes place, the. However, one is galvanic and one is electrolytic. this worksheet will cover galvanic cells and their components, such as anodes, cathodes, wires, salt bridges, and electrolyte. a galvanic cell or voltaic cell is a device in. The Galvanic Cell Worksheet.
From www.chegg.com
Solved Complete the worksheet given the following galvanic The Galvanic Cell Worksheet Make you indicate all of. The cells in problems 6 and 7 have almost the same components. a galvanic cell may have a negative or positive cell potential. As the reaction in this cell takes place, the. calculate the standard cell potential produced by a galvanic cell consisting of. However, one is galvanic and one is electrolytic. . The Galvanic Cell Worksheet.
From study.com
Quiz & Worksheet Electrolytic Cells vs. Galvanic Cells The Galvanic Cell Worksheet The cells in problems 6 and 7 have almost the same components. this worksheet will cover galvanic cells and their components, such as anodes, cathodes, wires, salt bridges, and electrolyte. Make you indicate all of. in this activity, students will use a simulation to create a variety of galvanic/voltaic cells with different. 6.the overall reaction in a. The Galvanic Cell Worksheet.
From www.studypool.com
SOLUTION G9 galvanic cell worksheet Studypool The Galvanic Cell Worksheet a galvanic cell or voltaic cell is a device in which a redox reaction, such as the one in equation (4), spontaneously occurs and. calculate the standard cell potential produced by a galvanic cell consisting of. this worksheet will cover galvanic cells and their components, such as anodes, cathodes, wires, salt bridges, and electrolyte. The cells in. The Galvanic Cell Worksheet.
From www.coursehero.com
[Solved] 3. The following is a diagram of a galvanic cell. The LEFT... Course Hero The Galvanic Cell Worksheet calculate the standard cell potential produced by a galvanic cell consisting of. in this activity, students will use a simulation to create a variety of galvanic/voltaic cells with different. The cells in problems 6 and 7 have almost the same components. a galvanic cell may have a negative or positive cell potential. 6.the overall reaction in. The Galvanic Cell Worksheet.
From www.pinterest.ca
Redox Reactions Notes and Galvanic Cells Redox reactions, Chemistry notes, Galvanic cell The Galvanic Cell Worksheet The cells in problems 6 and 7 have almost the same components. Make you indicate all of. a galvanic cell may have a negative or positive cell potential. As the reaction in this cell takes place, the. in this activity, students will use a simulation to create a variety of galvanic/voltaic cells with different. a galvanic cell. The Galvanic Cell Worksheet.
From www.studypool.com
SOLUTION CHEM 22 Galvanic Cell Worksheet Studypool The Galvanic Cell Worksheet Make you indicate all of. The cells in problems 6 and 7 have almost the same components. Cations always flow toward the anode. in this activity, students will use a simulation to create a variety of galvanic/voltaic cells with different. calculate the standard cell potential produced by a galvanic cell consisting of. this worksheet will cover galvanic. The Galvanic Cell Worksheet.
From studylib.net
Galvanic Cell Worksheet The Galvanic Cell Worksheet this worksheet will cover galvanic cells and their components, such as anodes, cathodes, wires, salt bridges, and electrolyte. As the reaction in this cell takes place, the. a galvanic cell or voltaic cell is a device in which a redox reaction, such as the one in equation (4), spontaneously occurs and. The cells in problems 6 and 7. The Galvanic Cell Worksheet.
From taylor-chapter.blogspot.com
Galvanic Cell Practice Problems With Answers 17+ Pages Summary Doc [2.1mb] Updated 2021 The Galvanic Cell Worksheet a galvanic cell may have a negative or positive cell potential. this worksheet will cover galvanic cells and their components, such as anodes, cathodes, wires, salt bridges, and electrolyte. a galvanic cell or voltaic cell is a device in which a redox reaction, such as the one in equation (4), spontaneously occurs and. in this activity,. The Galvanic Cell Worksheet.
From www.vrogue.co
Diagramming Galvanic Cells Handout And Worksheet Galv vrogue.co The Galvanic Cell Worksheet However, one is galvanic and one is electrolytic. The cells in problems 6 and 7 have almost the same components. Cations always flow toward the anode. this worksheet will cover galvanic cells and their components, such as anodes, cathodes, wires, salt bridges, and electrolyte. draw (graphically) a galvanic cell that takes advantage of the spontaneous redox reaction indicated. The Galvanic Cell Worksheet.
From www.studypool.com
SOLUTION G9 galvanic cell worksheet Studypool The Galvanic Cell Worksheet this worksheet will cover galvanic cells and their components, such as anodes, cathodes, wires, salt bridges, and electrolyte. draw (graphically) a galvanic cell that takes advantage of the spontaneous redox reaction indicated in q2. Cations always flow toward the anode. a galvanic cell may have a negative or positive cell potential. 6.the overall reaction in a. The Galvanic Cell Worksheet.
From www.studocu.com
Galvanic Cells selfmade chemistry worksheet Galvanic Cells Chemistry2e What is the The Galvanic Cell Worksheet in this activity, students will use a simulation to create a variety of galvanic/voltaic cells with different. this worksheet will cover galvanic cells and their components, such as anodes, cathodes, wires, salt bridges, and electrolyte. As the reaction in this cell takes place, the. calculate the standard cell potential produced by a galvanic cell consisting of. . The Galvanic Cell Worksheet.
From www.chemistrylearner.com
Galvanic Cell (Voltaic Cell) Chemistry Learner The Galvanic Cell Worksheet calculate the standard cell potential produced by a galvanic cell consisting of. 6.the overall reaction in a electrochemical cell is zn(s) + cu2+(aq) ® zn2+(aq) + cu(s). in this activity, students will use a simulation to create a variety of galvanic/voltaic cells with different. this worksheet will cover galvanic cells and their components, such as anodes,. The Galvanic Cell Worksheet.
From www.yumpu.com
Activity worksheets Questions ACTIVITY SHEET 1 1. A voltaic cell The Galvanic Cell Worksheet draw (graphically) a galvanic cell that takes advantage of the spontaneous redox reaction indicated in q2. Make you indicate all of. calculate the standard cell potential produced by a galvanic cell consisting of. in this activity, students will use a simulation to create a variety of galvanic/voltaic cells with different. The cells in problems 6 and 7. The Galvanic Cell Worksheet.
From ar.inspiredpencil.com
Galvanic Cell Equation The Galvanic Cell Worksheet in this activity, students will use a simulation to create a variety of galvanic/voltaic cells with different. this worksheet will cover galvanic cells and their components, such as anodes, cathodes, wires, salt bridges, and electrolyte. The cells in problems 6 and 7 have almost the same components. a galvanic cell or voltaic cell is a device in. The Galvanic Cell Worksheet.
From mungfali.com
Blank Galvanic Cell Diagram The Galvanic Cell Worksheet As the reaction in this cell takes place, the. draw (graphically) a galvanic cell that takes advantage of the spontaneous redox reaction indicated in q2. Make you indicate all of. The cells in problems 6 and 7 have almost the same components. However, one is galvanic and one is electrolytic. Cations always flow toward the anode. in this. The Galvanic Cell Worksheet.
From general.chemistrysteps.com
Galvanic Cells Chemistry Steps The Galvanic Cell Worksheet calculate the standard cell potential produced by a galvanic cell consisting of. a galvanic cell may have a negative or positive cell potential. draw (graphically) a galvanic cell that takes advantage of the spontaneous redox reaction indicated in q2. Cations always flow toward the anode. As the reaction in this cell takes place, the. Make you indicate. The Galvanic Cell Worksheet.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working The Galvanic Cell Worksheet in this activity, students will use a simulation to create a variety of galvanic/voltaic cells with different. However, one is galvanic and one is electrolytic. Cations always flow toward the anode. this worksheet will cover galvanic cells and their components, such as anodes, cathodes, wires, salt bridges, and electrolyte. The cells in problems 6 and 7 have almost. The Galvanic Cell Worksheet.
From www.liveworksheets.com
Galvanic Cell activity The Galvanic Cell Worksheet in this activity, students will use a simulation to create a variety of galvanic/voltaic cells with different. 6.the overall reaction in a electrochemical cell is zn(s) + cu2+(aq) ® zn2+(aq) + cu(s). a galvanic cell may have a negative or positive cell potential. The cells in problems 6 and 7 have almost the same components. Cations always. The Galvanic Cell Worksheet.
From www.studypool.com
SOLUTION G9 galvanic cell worksheet Studypool The Galvanic Cell Worksheet a galvanic cell may have a negative or positive cell potential. As the reaction in this cell takes place, the. calculate the standard cell potential produced by a galvanic cell consisting of. However, one is galvanic and one is electrolytic. Cations always flow toward the anode. The cells in problems 6 and 7 have almost the same components.. The Galvanic Cell Worksheet.
From www.vrogue.co
Diagramming Galvanic Cells Handout And Worksheet Galv vrogue.co The Galvanic Cell Worksheet As the reaction in this cell takes place, the. this worksheet will cover galvanic cells and their components, such as anodes, cathodes, wires, salt bridges, and electrolyte. Make you indicate all of. The cells in problems 6 and 7 have almost the same components. a galvanic cell or voltaic cell is a device in which a redox reaction,. The Galvanic Cell Worksheet.
From studylib.net
Electrolytic vs. Galvanic Cell Worksheet The Galvanic Cell Worksheet draw (graphically) a galvanic cell that takes advantage of the spontaneous redox reaction indicated in q2. Make you indicate all of. However, one is galvanic and one is electrolytic. As the reaction in this cell takes place, the. 6.the overall reaction in a electrochemical cell is zn(s) + cu2+(aq) ® zn2+(aq) + cu(s). calculate the standard cell. The Galvanic Cell Worksheet.
From www.liveworksheets.com
Galvanic cell interactive worksheet Live Worksheets The Galvanic Cell Worksheet in this activity, students will use a simulation to create a variety of galvanic/voltaic cells with different. this worksheet will cover galvanic cells and their components, such as anodes, cathodes, wires, salt bridges, and electrolyte. 6.the overall reaction in a electrochemical cell is zn(s) + cu2+(aq) ® zn2+(aq) + cu(s). As the reaction in this cell takes. The Galvanic Cell Worksheet.
From www.chegg.com
Solved A galvanic cell is prepared with tin and silver The Galvanic Cell Worksheet As the reaction in this cell takes place, the. draw (graphically) a galvanic cell that takes advantage of the spontaneous redox reaction indicated in q2. Cations always flow toward the anode. The cells in problems 6 and 7 have almost the same components. 6.the overall reaction in a electrochemical cell is zn(s) + cu2+(aq) ® zn2+(aq) + cu(s).. The Galvanic Cell Worksheet.