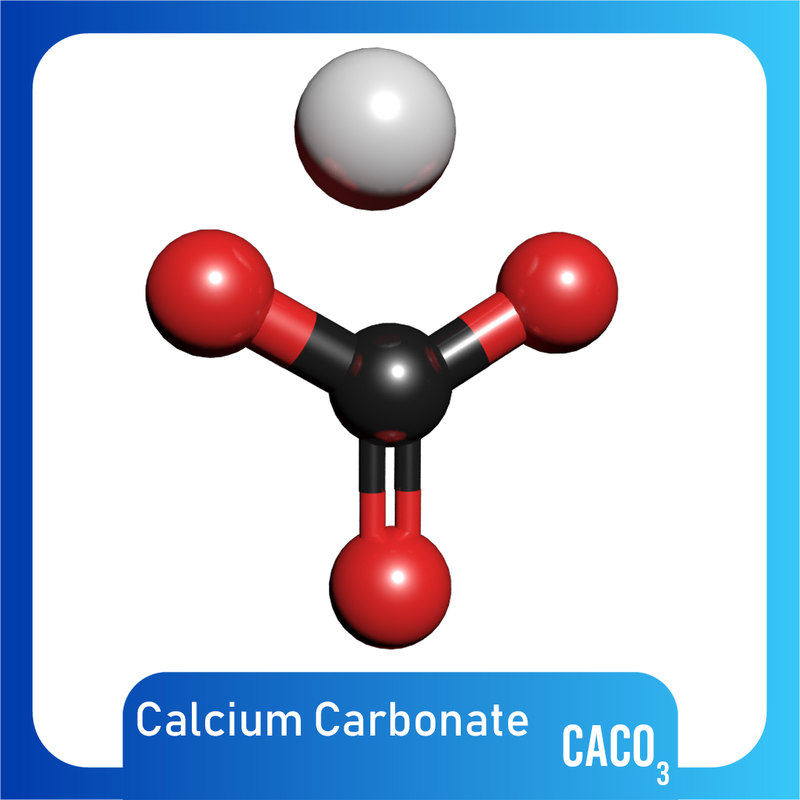
What Is Molecular Structure Of Caco3 . Calcium carbonate (caco3), chemical compound consisting of one atom of calcium, one of carbon, and three of oxygen that is the major constituent of limestone, marble, chalk, eggshells, bivalve shells, and corals. At its core, this compound consists of one calcium (ca) ion bonded to one. Calcium carbonate is either a white powder or a colorless crystal. Other forms are possible to be made, the denser 2.83g/cm3. = 40.08 + 12.01 + 48.00 = 40.08+12.01+48.00 = 100.09 g/mol. From a chemical standpoint, calcium carbonate is a base that reacts with acids to form calcium salts, water, and carbon dioxide. The thermodynamically stable structure of caco 3 is hexagonal β − caco3 (the mineral calcite) under normal conditions. Molar mass of caco 3 = 40.08+12.01+3×16.00. Calcium carbonate is characterized by its unique chemical structure, represented by the formula caco₃. Visit byju's to understand the properties, structure and uses.
from www.turbosquid.com
Other forms are possible to be made, the denser 2.83g/cm3. At its core, this compound consists of one calcium (ca) ion bonded to one. From a chemical standpoint, calcium carbonate is a base that reacts with acids to form calcium salts, water, and carbon dioxide. Visit byju's to understand the properties, structure and uses. Calcium carbonate is either a white powder or a colorless crystal. Calcium carbonate is characterized by its unique chemical structure, represented by the formula caco₃. = 40.08 + 12.01 + 48.00 = 40.08+12.01+48.00 = 100.09 g/mol. Molar mass of caco 3 = 40.08+12.01+3×16.00. Calcium carbonate (caco3), chemical compound consisting of one atom of calcium, one of carbon, and three of oxygen that is the major constituent of limestone, marble, chalk, eggshells, bivalve shells, and corals. The thermodynamically stable structure of caco 3 is hexagonal β − caco3 (the mineral calcite) under normal conditions.
Caco3 calcium carbonate 3D model TurboSquid 1423526
What Is Molecular Structure Of Caco3 Visit byju's to understand the properties, structure and uses. At its core, this compound consists of one calcium (ca) ion bonded to one. = 40.08 + 12.01 + 48.00 = 40.08+12.01+48.00 = 100.09 g/mol. From a chemical standpoint, calcium carbonate is a base that reacts with acids to form calcium salts, water, and carbon dioxide. Molar mass of caco 3 = 40.08+12.01+3×16.00. Calcium carbonate is either a white powder or a colorless crystal. Other forms are possible to be made, the denser 2.83g/cm3. The thermodynamically stable structure of caco 3 is hexagonal β − caco3 (the mineral calcite) under normal conditions. Visit byju's to understand the properties, structure and uses. Calcium carbonate is characterized by its unique chemical structure, represented by the formula caco₃. Calcium carbonate (caco3), chemical compound consisting of one atom of calcium, one of carbon, and three of oxygen that is the major constituent of limestone, marble, chalk, eggshells, bivalve shells, and corals.
From free3d.com
Calcium Carbonate 3D Model CaCO3 3D Model 5 .unknown .3ds .fbx .max What Is Molecular Structure Of Caco3 = 40.08 + 12.01 + 48.00 = 40.08+12.01+48.00 = 100.09 g/mol. Calcium carbonate is either a white powder or a colorless crystal. Calcium carbonate (caco3), chemical compound consisting of one atom of calcium, one of carbon, and three of oxygen that is the major constituent of limestone, marble, chalk, eggshells, bivalve shells, and corals. At its core, this compound consists. What Is Molecular Structure Of Caco3.
From www.adda247.com
CaCO3 Chemical & Common Name, Molar & Atomic Mass What Is Molecular Structure Of Caco3 From a chemical standpoint, calcium carbonate is a base that reacts with acids to form calcium salts, water, and carbon dioxide. Other forms are possible to be made, the denser 2.83g/cm3. The thermodynamically stable structure of caco 3 is hexagonal β − caco3 (the mineral calcite) under normal conditions. Calcium carbonate is characterized by its unique chemical structure, represented by. What Is Molecular Structure Of Caco3.
From ar.inspiredpencil.com
Caco3 Molecular Structure What Is Molecular Structure Of Caco3 Calcium carbonate (caco3), chemical compound consisting of one atom of calcium, one of carbon, and three of oxygen that is the major constituent of limestone, marble, chalk, eggshells, bivalve shells, and corals. Other forms are possible to be made, the denser 2.83g/cm3. Molar mass of caco 3 = 40.08+12.01+3×16.00. From a chemical standpoint, calcium carbonate is a base that reacts. What Is Molecular Structure Of Caco3.
From www.esrf.fr
Manganese incorporation into mussel shells elucidated using Xray What Is Molecular Structure Of Caco3 Other forms are possible to be made, the denser 2.83g/cm3. At its core, this compound consists of one calcium (ca) ion bonded to one. Molar mass of caco 3 = 40.08+12.01+3×16.00. Calcium carbonate is either a white powder or a colorless crystal. From a chemical standpoint, calcium carbonate is a base that reacts with acids to form calcium salts, water,. What Is Molecular Structure Of Caco3.
From brainly.in
What mass of caco3 is required to react completely with 25 ml of 0.75m What Is Molecular Structure Of Caco3 Calcium carbonate is either a white powder or a colorless crystal. Visit byju's to understand the properties, structure and uses. Calcium carbonate is characterized by its unique chemical structure, represented by the formula caco₃. Calcium carbonate (caco3), chemical compound consisting of one atom of calcium, one of carbon, and three of oxygen that is the major constituent of limestone, marble,. What Is Molecular Structure Of Caco3.
From www.youtube.com
Molar mass CaCO3Calculate molecular weight Calcium carbonateCalcium What Is Molecular Structure Of Caco3 The thermodynamically stable structure of caco 3 is hexagonal β − caco3 (the mineral calcite) under normal conditions. From a chemical standpoint, calcium carbonate is a base that reacts with acids to form calcium salts, water, and carbon dioxide. Visit byju's to understand the properties, structure and uses. Other forms are possible to be made, the denser 2.83g/cm3. = 40.08. What Is Molecular Structure Of Caco3.
From gbu-taganskij.ru
Calcium Carbonate 3D Model CaCO3 3D Model 5 Free3D, 44 OFF What Is Molecular Structure Of Caco3 Calcium carbonate is either a white powder or a colorless crystal. Visit byju's to understand the properties, structure and uses. At its core, this compound consists of one calcium (ca) ion bonded to one. = 40.08 + 12.01 + 48.00 = 40.08+12.01+48.00 = 100.09 g/mol. The thermodynamically stable structure of caco 3 is hexagonal β − caco3 (the mineral calcite). What Is Molecular Structure Of Caco3.
From www.researchgate.net
Scheme 1. Schematic illustration of the synthesis of (a) CaCO3 core What Is Molecular Structure Of Caco3 Other forms are possible to be made, the denser 2.83g/cm3. Calcium carbonate is characterized by its unique chemical structure, represented by the formula caco₃. Calcium carbonate (caco3), chemical compound consisting of one atom of calcium, one of carbon, and three of oxygen that is the major constituent of limestone, marble, chalk, eggshells, bivalve shells, and corals. The thermodynamically stable structure. What Is Molecular Structure Of Caco3.
From cartoondealer.com
Calcium Carbonate Molecule 3d Rendering, Flat Molecular Structure With What Is Molecular Structure Of Caco3 Calcium carbonate is characterized by its unique chemical structure, represented by the formula caco₃. Visit byju's to understand the properties, structure and uses. = 40.08 + 12.01 + 48.00 = 40.08+12.01+48.00 = 100.09 g/mol. Molar mass of caco 3 = 40.08+12.01+3×16.00. Other forms are possible to be made, the denser 2.83g/cm3. From a chemical standpoint, calcium carbonate is a base. What Is Molecular Structure Of Caco3.
From www.youtube.com
Is CaCO3 (Calcium carbonate) Ionic or Covalent? YouTube What Is Molecular Structure Of Caco3 Other forms are possible to be made, the denser 2.83g/cm3. Calcium carbonate (caco3), chemical compound consisting of one atom of calcium, one of carbon, and three of oxygen that is the major constituent of limestone, marble, chalk, eggshells, bivalve shells, and corals. At its core, this compound consists of one calcium (ca) ion bonded to one. Calcium carbonate is either. What Is Molecular Structure Of Caco3.
From www.chegg.com
Solved A 14.549 g sample of CaCl, was added to 28.039 g of What Is Molecular Structure Of Caco3 At its core, this compound consists of one calcium (ca) ion bonded to one. = 40.08 + 12.01 + 48.00 = 40.08+12.01+48.00 = 100.09 g/mol. Calcium carbonate is either a white powder or a colorless crystal. Calcium carbonate is characterized by its unique chemical structure, represented by the formula caco₃. Calcium carbonate (caco3), chemical compound consisting of one atom of. What Is Molecular Structure Of Caco3.
From www.pngegg.com
Diagrama de carbonato de calcio estructura lewis química, california What Is Molecular Structure Of Caco3 The thermodynamically stable structure of caco 3 is hexagonal β − caco3 (the mineral calcite) under normal conditions. Calcium carbonate is characterized by its unique chemical structure, represented by the formula caco₃. = 40.08 + 12.01 + 48.00 = 40.08+12.01+48.00 = 100.09 g/mol. From a chemical standpoint, calcium carbonate is a base that reacts with acids to form calcium salts,. What Is Molecular Structure Of Caco3.
From bg.puntomarinero.com
CaCO3 разлагане, подготовка, химични свойства, приложение What Is Molecular Structure Of Caco3 From a chemical standpoint, calcium carbonate is a base that reacts with acids to form calcium salts, water, and carbon dioxide. At its core, this compound consists of one calcium (ca) ion bonded to one. The thermodynamically stable structure of caco 3 is hexagonal β − caco3 (the mineral calcite) under normal conditions. Visit byju's to understand the properties, structure. What Is Molecular Structure Of Caco3.
From brainly.in
What mass of 95 pure caco3 will be required to neutralise 50 ml of 0.5 What Is Molecular Structure Of Caco3 From a chemical standpoint, calcium carbonate is a base that reacts with acids to form calcium salts, water, and carbon dioxide. Calcium carbonate is characterized by its unique chemical structure, represented by the formula caco₃. = 40.08 + 12.01 + 48.00 = 40.08+12.01+48.00 = 100.09 g/mol. Molar mass of caco 3 = 40.08+12.01+3×16.00. The thermodynamically stable structure of caco 3. What Is Molecular Structure Of Caco3.
From askfilo.com
What is molecular mass of CaCO3 ? Filo What Is Molecular Structure Of Caco3 Molar mass of caco 3 = 40.08+12.01+3×16.00. Calcium carbonate is characterized by its unique chemical structure, represented by the formula caco₃. Visit byju's to understand the properties, structure and uses. From a chemical standpoint, calcium carbonate is a base that reacts with acids to form calcium salts, water, and carbon dioxide. Calcium carbonate is either a white powder or a. What Is Molecular Structure Of Caco3.
From www.youtube.com
Calcium carbonate YouTube What Is Molecular Structure Of Caco3 Calcium carbonate is characterized by its unique chemical structure, represented by the formula caco₃. Other forms are possible to be made, the denser 2.83g/cm3. Molar mass of caco 3 = 40.08+12.01+3×16.00. At its core, this compound consists of one calcium (ca) ion bonded to one. From a chemical standpoint, calcium carbonate is a base that reacts with acids to form. What Is Molecular Structure Of Caco3.
From brainly.in
The equivalent weight of Ca in CaCO3 isdo it with complete explanation What Is Molecular Structure Of Caco3 The thermodynamically stable structure of caco 3 is hexagonal β − caco3 (the mineral calcite) under normal conditions. Calcium carbonate is either a white powder or a colorless crystal. = 40.08 + 12.01 + 48.00 = 40.08+12.01+48.00 = 100.09 g/mol. Calcium carbonate (caco3), chemical compound consisting of one atom of calcium, one of carbon, and three of oxygen that is. What Is Molecular Structure Of Caco3.
From www.thoughtco.com
Compounds With Ionic and Covalent Bonds What Is Molecular Structure Of Caco3 Molar mass of caco 3 = 40.08+12.01+3×16.00. Calcium carbonate is either a white powder or a colorless crystal. Other forms are possible to be made, the denser 2.83g/cm3. = 40.08 + 12.01 + 48.00 = 40.08+12.01+48.00 = 100.09 g/mol. Calcium carbonate is characterized by its unique chemical structure, represented by the formula caco₃. From a chemical standpoint, calcium carbonate is. What Is Molecular Structure Of Caco3.
From www.youtube.com
How to draw Lewis structure of CaCO3Nelson chemistry CaCO3 What Is Molecular Structure Of Caco3 Other forms are possible to be made, the denser 2.83g/cm3. Calcium carbonate is either a white powder or a colorless crystal. Molar mass of caco 3 = 40.08+12.01+3×16.00. At its core, this compound consists of one calcium (ca) ion bonded to one. Calcium carbonate (caco3), chemical compound consisting of one atom of calcium, one of carbon, and three of oxygen. What Is Molecular Structure Of Caco3.
From www.turbosquid.com
Caco3 calcium carbonate 3D model TurboSquid 1423526 What Is Molecular Structure Of Caco3 At its core, this compound consists of one calcium (ca) ion bonded to one. = 40.08 + 12.01 + 48.00 = 40.08+12.01+48.00 = 100.09 g/mol. Visit byju's to understand the properties, structure and uses. From a chemical standpoint, calcium carbonate is a base that reacts with acids to form calcium salts, water, and carbon dioxide. Molar mass of caco 3. What Is Molecular Structure Of Caco3.
From gbu-taganskij.ru
CaCO3 Lewis Structure Characteristics 15 Complete Facts, 56 OFF What Is Molecular Structure Of Caco3 Calcium carbonate is characterized by its unique chemical structure, represented by the formula caco₃. Other forms are possible to be made, the denser 2.83g/cm3. From a chemical standpoint, calcium carbonate is a base that reacts with acids to form calcium salts, water, and carbon dioxide. Calcium carbonate is either a white powder or a colorless crystal. = 40.08 + 12.01. What Is Molecular Structure Of Caco3.
From ar.inspiredpencil.com
Caco3 Structure What Is Molecular Structure Of Caco3 At its core, this compound consists of one calcium (ca) ion bonded to one. Molar mass of caco 3 = 40.08+12.01+3×16.00. = 40.08 + 12.01 + 48.00 = 40.08+12.01+48.00 = 100.09 g/mol. Other forms are possible to be made, the denser 2.83g/cm3. Calcium carbonate is characterized by its unique chemical structure, represented by the formula caco₃. From a chemical standpoint,. What Is Molecular Structure Of Caco3.
From ar.inspiredpencil.com
Calcium Carbonate Molecular Structure What Is Molecular Structure Of Caco3 From a chemical standpoint, calcium carbonate is a base that reacts with acids to form calcium salts, water, and carbon dioxide. Other forms are possible to be made, the denser 2.83g/cm3. At its core, this compound consists of one calcium (ca) ion bonded to one. Calcium carbonate (caco3), chemical compound consisting of one atom of calcium, one of carbon, and. What Is Molecular Structure Of Caco3.
From www.chegg.com
Solved 2.10 (a) What is the molecular weight of CaCO3 ? (b) What Is Molecular Structure Of Caco3 Calcium carbonate is characterized by its unique chemical structure, represented by the formula caco₃. = 40.08 + 12.01 + 48.00 = 40.08+12.01+48.00 = 100.09 g/mol. Visit byju's to understand the properties, structure and uses. The thermodynamically stable structure of caco 3 is hexagonal β − caco3 (the mineral calcite) under normal conditions. Calcium carbonate is either a white powder or. What Is Molecular Structure Of Caco3.
From ar.inspiredpencil.com
Caco3 Molecular Structure What Is Molecular Structure Of Caco3 The thermodynamically stable structure of caco 3 is hexagonal β − caco3 (the mineral calcite) under normal conditions. = 40.08 + 12.01 + 48.00 = 40.08+12.01+48.00 = 100.09 g/mol. Calcium carbonate is characterized by its unique chemical structure, represented by the formula caco₃. Other forms are possible to be made, the denser 2.83g/cm3. From a chemical standpoint, calcium carbonate is. What Is Molecular Structure Of Caco3.
From ar.inspiredpencil.com
Calcium Carbonate Formula What Is Molecular Structure Of Caco3 = 40.08 + 12.01 + 48.00 = 40.08+12.01+48.00 = 100.09 g/mol. Molar mass of caco 3 = 40.08+12.01+3×16.00. Other forms are possible to be made, the denser 2.83g/cm3. Calcium carbonate is characterized by its unique chemical structure, represented by the formula caco₃. Calcium carbonate (caco3), chemical compound consisting of one atom of calcium, one of carbon, and three of oxygen. What Is Molecular Structure Of Caco3.
From ar.inspiredpencil.com
Caco3 Molecular Structure What Is Molecular Structure Of Caco3 = 40.08 + 12.01 + 48.00 = 40.08+12.01+48.00 = 100.09 g/mol. Visit byju's to understand the properties, structure and uses. Molar mass of caco 3 = 40.08+12.01+3×16.00. Calcium carbonate (caco3), chemical compound consisting of one atom of calcium, one of carbon, and three of oxygen that is the major constituent of limestone, marble, chalk, eggshells, bivalve shells, and corals. Other. What Is Molecular Structure Of Caco3.
From zhidao.baidu.com
请问有那个高手知道碳酸钙的分子结构示意图,方便一下,谢谢_百度知道 What Is Molecular Structure Of Caco3 = 40.08 + 12.01 + 48.00 = 40.08+12.01+48.00 = 100.09 g/mol. At its core, this compound consists of one calcium (ca) ion bonded to one. From a chemical standpoint, calcium carbonate is a base that reacts with acids to form calcium salts, water, and carbon dioxide. Calcium carbonate is either a white powder or a colorless crystal. Molar mass of. What Is Molecular Structure Of Caco3.
From socratic.org
When are the bonds of ions both covalent and ionic? Socratic What Is Molecular Structure Of Caco3 Calcium carbonate is either a white powder or a colorless crystal. Molar mass of caco 3 = 40.08+12.01+3×16.00. At its core, this compound consists of one calcium (ca) ion bonded to one. The thermodynamically stable structure of caco 3 is hexagonal β − caco3 (the mineral calcite) under normal conditions. Calcium carbonate is characterized by its unique chemical structure, represented. What Is Molecular Structure Of Caco3.
From www.animalia-life.club
Calcium Carbonate Molecule What Is Molecular Structure Of Caco3 Calcium carbonate is characterized by its unique chemical structure, represented by the formula caco₃. Other forms are possible to be made, the denser 2.83g/cm3. The thermodynamically stable structure of caco 3 is hexagonal β − caco3 (the mineral calcite) under normal conditions. Calcium carbonate is either a white powder or a colorless crystal. = 40.08 + 12.01 + 48.00 =. What Is Molecular Structure Of Caco3.
From mungfali.com
CaCO3 Structure What Is Molecular Structure Of Caco3 Visit byju's to understand the properties, structure and uses. At its core, this compound consists of one calcium (ca) ion bonded to one. Calcium carbonate is characterized by its unique chemical structure, represented by the formula caco₃. = 40.08 + 12.01 + 48.00 = 40.08+12.01+48.00 = 100.09 g/mol. Calcium carbonate is either a white powder or a colorless crystal. The. What Is Molecular Structure Of Caco3.
From www.turbosquid.com
Caco3 calcium carbonate 3D model TurboSquid 1423526 What Is Molecular Structure Of Caco3 = 40.08 + 12.01 + 48.00 = 40.08+12.01+48.00 = 100.09 g/mol. Calcium carbonate (caco3), chemical compound consisting of one atom of calcium, one of carbon, and three of oxygen that is the major constituent of limestone, marble, chalk, eggshells, bivalve shells, and corals. Calcium carbonate is characterized by its unique chemical structure, represented by the formula caco₃. Molar mass of. What Is Molecular Structure Of Caco3.
From www.gamgi.org
GAMGI Screenshots > GAMGI 0.10 What Is Molecular Structure Of Caco3 Other forms are possible to be made, the denser 2.83g/cm3. The thermodynamically stable structure of caco 3 is hexagonal β − caco3 (the mineral calcite) under normal conditions. Calcium carbonate is characterized by its unique chemical structure, represented by the formula caco₃. Molar mass of caco 3 = 40.08+12.01+3×16.00. Calcium carbonate is either a white powder or a colorless crystal.. What Is Molecular Structure Of Caco3.
From www.vectorstock.com
Molecular structure calcium carbonate caco3 Vector Image What Is Molecular Structure Of Caco3 The thermodynamically stable structure of caco 3 is hexagonal β − caco3 (the mineral calcite) under normal conditions. Other forms are possible to be made, the denser 2.83g/cm3. Calcium carbonate is characterized by its unique chemical structure, represented by the formula caco₃. Visit byju's to understand the properties, structure and uses. Calcium carbonate is either a white powder or a. What Is Molecular Structure Of Caco3.
From solvedlib.com
In the reaction CaCO3(s) + 2HCl(aq) ==>CaCl2(aq) +… SolvedLib What Is Molecular Structure Of Caco3 The thermodynamically stable structure of caco 3 is hexagonal β − caco3 (the mineral calcite) under normal conditions. Calcium carbonate is either a white powder or a colorless crystal. Calcium carbonate is characterized by its unique chemical structure, represented by the formula caco₃. At its core, this compound consists of one calcium (ca) ion bonded to one. Visit byju's to. What Is Molecular Structure Of Caco3.