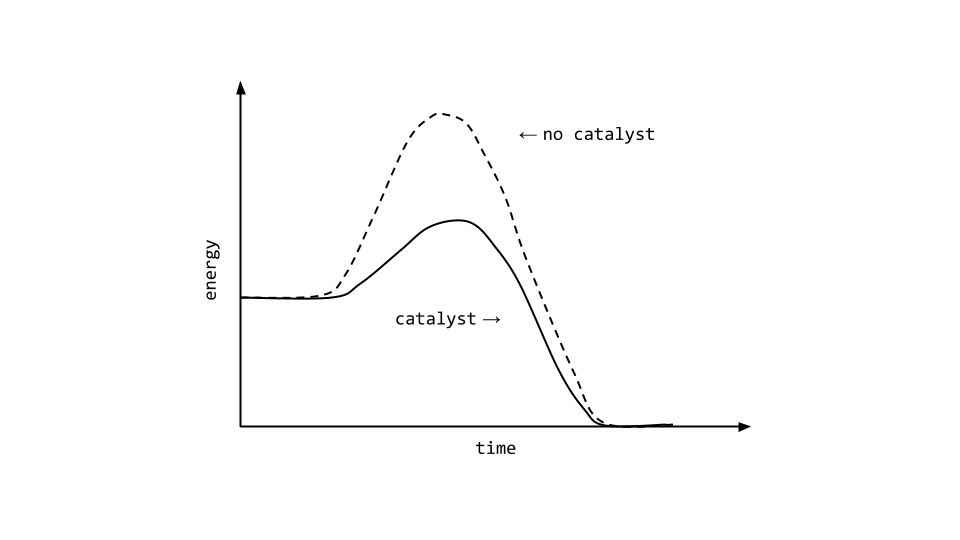
Difference Of Threshold Energy And Activation Energy . activation energy is the minimum amount of energy that must be available to reactants for a chemical reaction to occur. Threshold energy is the minimum energy. — activation energy plays a crucial role in determining the rate of a chemical reaction; Learn how activation energy affects. Learn how activation energy relates. learn the difference between threshold energy and activation energy, two concepts in chemical kinetics. learn the difference between activation energy and threshold energy, two fundamental concepts in chemistry and. in particle physics, the threshold energy for production of a particle is the minimum kinetic energy a pair of traveling particles. Activation energy is the minimum energy required for reactants to collide. — learn the difference between threshold energy and activation energy in chemical reactions. activation energy is the minimum energy required for a chemical reaction to occur. learn how to calculate activation energy and how it affects reaction rates.
from nesslabs.com
activation energy is the minimum energy required for a chemical reaction to occur. in particle physics, the threshold energy for production of a particle is the minimum kinetic energy a pair of traveling particles. — activation energy plays a crucial role in determining the rate of a chemical reaction; Learn how activation energy affects. learn the difference between activation energy and threshold energy, two fundamental concepts in chemistry and. Learn how activation energy relates. activation energy is the minimum amount of energy that must be available to reactants for a chemical reaction to occur. Activation energy is the minimum energy required for reactants to collide. learn how to calculate activation energy and how it affects reaction rates. Threshold energy is the minimum energy.
Activation energy the chemistry of getting started Ness Labs
Difference Of Threshold Energy And Activation Energy Activation energy is the minimum energy required for reactants to collide. learn how to calculate activation energy and how it affects reaction rates. Activation energy is the minimum energy required for reactants to collide. Learn how activation energy relates. — learn the difference between threshold energy and activation energy in chemical reactions. learn the difference between threshold energy and activation energy, two concepts in chemical kinetics. activation energy is the minimum amount of energy that must be available to reactants for a chemical reaction to occur. — activation energy plays a crucial role in determining the rate of a chemical reaction; in particle physics, the threshold energy for production of a particle is the minimum kinetic energy a pair of traveling particles. activation energy is the minimum energy required for a chemical reaction to occur. Threshold energy is the minimum energy. Learn how activation energy affects. learn the difference between activation energy and threshold energy, two fundamental concepts in chemistry and.
From www.slideserve.com
PPT Chapter 7 PowerPoint Presentation, free download ID239015 Difference Of Threshold Energy And Activation Energy learn the difference between threshold energy and activation energy, two concepts in chemical kinetics. activation energy is the minimum energy required for a chemical reaction to occur. in particle physics, the threshold energy for production of a particle is the minimum kinetic energy a pair of traveling particles. — learn the difference between threshold energy and. Difference Of Threshold Energy And Activation Energy.
From pediaa.com
Difference Between Energy and Activation Energy Definition, Forms of Difference Of Threshold Energy And Activation Energy learn the difference between activation energy and threshold energy, two fundamental concepts in chemistry and. — activation energy plays a crucial role in determining the rate of a chemical reaction; learn the difference between threshold energy and activation energy, two concepts in chemical kinetics. activation energy is the minimum amount of energy that must be available. Difference Of Threshold Energy And Activation Energy.
From byjus.com
26. What is difference between threshold energy and activation energy? Difference Of Threshold Energy And Activation Energy activation energy is the minimum energy required for a chemical reaction to occur. learn how to calculate activation energy and how it affects reaction rates. learn the difference between activation energy and threshold energy, two fundamental concepts in chemistry and. in particle physics, the threshold energy for production of a particle is the minimum kinetic energy. Difference Of Threshold Energy And Activation Energy.
From byjus.com
The forward and backward activation energy of exothermic reaction A→ B Difference Of Threshold Energy And Activation Energy activation energy is the minimum energy required for a chemical reaction to occur. — learn the difference between threshold energy and activation energy in chemical reactions. Threshold energy is the minimum energy. learn the difference between activation energy and threshold energy, two fundamental concepts in chemistry and. activation energy is the minimum amount of energy that. Difference Of Threshold Energy And Activation Energy.
From classnotes.org.in
Arrhenius Equation and Activation Energy Chemical Chemistry Difference Of Threshold Energy And Activation Energy learn how to calculate activation energy and how it affects reaction rates. — learn the difference between threshold energy and activation energy in chemical reactions. Threshold energy is the minimum energy. activation energy is the minimum energy required for a chemical reaction to occur. — activation energy plays a crucial role in determining the rate of. Difference Of Threshold Energy And Activation Energy.
From www.youtube.com
25) Activation Energy and Threshold Energy Class12 Chemical Difference Of Threshold Energy And Activation Energy in particle physics, the threshold energy for production of a particle is the minimum kinetic energy a pair of traveling particles. learn the difference between threshold energy and activation energy, two concepts in chemical kinetics. Activation energy is the minimum energy required for reactants to collide. Threshold energy is the minimum energy. learn how to calculate activation. Difference Of Threshold Energy And Activation Energy.
From www.expii.com
Energy Diagram — Overview & Parts Expii Difference Of Threshold Energy And Activation Energy learn the difference between threshold energy and activation energy, two concepts in chemical kinetics. — activation energy plays a crucial role in determining the rate of a chemical reaction; activation energy is the minimum amount of energy that must be available to reactants for a chemical reaction to occur. learn how to calculate activation energy and. Difference Of Threshold Energy And Activation Energy.
From www.youtube.com
What is activation energy Threshold energy energy barrier rate of Difference Of Threshold Energy And Activation Energy Learn how activation energy affects. learn how to calculate activation energy and how it affects reaction rates. — learn the difference between threshold energy and activation energy in chemical reactions. Threshold energy is the minimum energy. Learn how activation energy relates. learn the difference between activation energy and threshold energy, two fundamental concepts in chemistry and. . Difference Of Threshold Energy And Activation Energy.
From sciencenotes.org
What Is Activation Energy? Definition and Examples Difference Of Threshold Energy And Activation Energy activation energy is the minimum amount of energy that must be available to reactants for a chemical reaction to occur. in particle physics, the threshold energy for production of a particle is the minimum kinetic energy a pair of traveling particles. learn the difference between threshold energy and activation energy, two concepts in chemical kinetics. —. Difference Of Threshold Energy And Activation Energy.
From www.chemistrylearner.com
Activation Energy Definition, Formula, and Graph Difference Of Threshold Energy And Activation Energy Learn how activation energy affects. Learn how activation energy relates. learn how to calculate activation energy and how it affects reaction rates. activation energy is the minimum energy required for a chemical reaction to occur. — activation energy plays a crucial role in determining the rate of a chemical reaction; Activation energy is the minimum energy required. Difference Of Threshold Energy And Activation Energy.
From www.kosmotime.com
Activation Energy The Secret to Getting Started and Getting Finished Difference Of Threshold Energy And Activation Energy Learn how activation energy relates. Activation energy is the minimum energy required for reactants to collide. — learn the difference between threshold energy and activation energy in chemical reactions. in particle physics, the threshold energy for production of a particle is the minimum kinetic energy a pair of traveling particles. learn the difference between activation energy and. Difference Of Threshold Energy And Activation Energy.
From byjus.com
What happens when the energy of a reaction if more than threshold Difference Of Threshold Energy And Activation Energy activation energy is the minimum amount of energy that must be available to reactants for a chemical reaction to occur. learn the difference between threshold energy and activation energy, two concepts in chemical kinetics. Learn how activation energy relates. Threshold energy is the minimum energy. — activation energy plays a crucial role in determining the rate of. Difference Of Threshold Energy And Activation Energy.
From www.youtube.com
Relationship between activation energy and reaction rate Reaction Difference Of Threshold Energy And Activation Energy learn the difference between activation energy and threshold energy, two fundamental concepts in chemistry and. — activation energy plays a crucial role in determining the rate of a chemical reaction; activation energy is the minimum energy required for a chemical reaction to occur. activation energy is the minimum amount of energy that must be available to. Difference Of Threshold Energy And Activation Energy.
From www.logiota.com
Activation Energy Chemical Physical Chemistry Chemistry Difference Of Threshold Energy And Activation Energy learn how to calculate activation energy and how it affects reaction rates. Threshold energy is the minimum energy. Activation energy is the minimum energy required for reactants to collide. Learn how activation energy affects. learn the difference between threshold energy and activation energy, two concepts in chemical kinetics. activation energy is the minimum amount of energy that. Difference Of Threshold Energy And Activation Energy.
From byjus.com
Activation Energy Definition, Formula, SI Units, Examples, Calculation Difference Of Threshold Energy And Activation Energy — activation energy plays a crucial role in determining the rate of a chemical reaction; Learn how activation energy relates. Activation energy is the minimum energy required for reactants to collide. activation energy is the minimum energy required for a chemical reaction to occur. — learn the difference between threshold energy and activation energy in chemical reactions.. Difference Of Threshold Energy And Activation Energy.
From www.differencebetween.com
Difference Between Energy and Activation Energy Compare the Difference Of Threshold Energy And Activation Energy learn how to calculate activation energy and how it affects reaction rates. — activation energy plays a crucial role in determining the rate of a chemical reaction; Activation energy is the minimum energy required for reactants to collide. Learn how activation energy affects. activation energy is the minimum energy required for a chemical reaction to occur. Threshold. Difference Of Threshold Energy And Activation Energy.
From edurev.in
Difference between activation energy and threshold energy I know the Difference Of Threshold Energy And Activation Energy activation energy is the minimum amount of energy that must be available to reactants for a chemical reaction to occur. — learn the difference between threshold energy and activation energy in chemical reactions. learn the difference between activation energy and threshold energy, two fundamental concepts in chemistry and. Activation energy is the minimum energy required for reactants. Difference Of Threshold Energy And Activation Energy.
From www.alamy.com
Graph of Progress of Reaction and Threshold energy Stock Vector Image Difference Of Threshold Energy And Activation Energy — learn the difference between threshold energy and activation energy in chemical reactions. Activation energy is the minimum energy required for reactants to collide. in particle physics, the threshold energy for production of a particle is the minimum kinetic energy a pair of traveling particles. learn the difference between threshold energy and activation energy, two concepts in. Difference Of Threshold Energy And Activation Energy.
From byjus.com
What happens when the energy of a reaction if more than threshold Difference Of Threshold Energy And Activation Energy learn the difference between activation energy and threshold energy, two fundamental concepts in chemistry and. Activation energy is the minimum energy required for reactants to collide. — activation energy plays a crucial role in determining the rate of a chemical reaction; activation energy is the minimum amount of energy that must be available to reactants for a. Difference Of Threshold Energy And Activation Energy.
From www.youtube.com
Difference between Activation energy and Threshold energy YouTube Difference Of Threshold Energy And Activation Energy — learn the difference between threshold energy and activation energy in chemical reactions. Threshold energy is the minimum energy. Learn how activation energy affects. — activation energy plays a crucial role in determining the rate of a chemical reaction; activation energy is the minimum energy required for a chemical reaction to occur. learn the difference between. Difference Of Threshold Energy And Activation Energy.
From www.differencebetween.com
Difference Between Activation Energy and Threshold Energy Compare the Difference Of Threshold Energy And Activation Energy Threshold energy is the minimum energy. activation energy is the minimum energy required for a chemical reaction to occur. activation energy is the minimum amount of energy that must be available to reactants for a chemical reaction to occur. learn the difference between threshold energy and activation energy, two concepts in chemical kinetics. Learn how activation energy. Difference Of Threshold Energy And Activation Energy.
From bio.libretexts.org
3.9 Energy in Chemical Reactions Biology LibreTexts Difference Of Threshold Energy And Activation Energy — learn the difference between threshold energy and activation energy in chemical reactions. Threshold energy is the minimum energy. — activation energy plays a crucial role in determining the rate of a chemical reaction; Learn how activation energy relates. learn the difference between threshold energy and activation energy, two concepts in chemical kinetics. in particle physics,. Difference Of Threshold Energy And Activation Energy.
From nesslabs.com
Activation energy the chemistry of getting started Ness Labs Difference Of Threshold Energy And Activation Energy Learn how activation energy affects. — activation energy plays a crucial role in determining the rate of a chemical reaction; Activation energy is the minimum energy required for reactants to collide. activation energy is the minimum amount of energy that must be available to reactants for a chemical reaction to occur. activation energy is the minimum energy. Difference Of Threshold Energy And Activation Energy.
From blogs.glowscotland.org.uk
Activation Energy Higher Chemistry Unit 1 Difference Of Threshold Energy And Activation Energy learn how to calculate activation energy and how it affects reaction rates. Learn how activation energy affects. Activation energy is the minimum energy required for reactants to collide. Threshold energy is the minimum energy. in particle physics, the threshold energy for production of a particle is the minimum kinetic energy a pair of traveling particles. — activation. Difference Of Threshold Energy And Activation Energy.
From wiredataisanewlk.z4.web.core.windows.net
How To Read Energy Diagrams Chemistry Difference Of Threshold Energy And Activation Energy — learn the difference between threshold energy and activation energy in chemical reactions. learn how to calculate activation energy and how it affects reaction rates. Learn how activation energy affects. learn the difference between activation energy and threshold energy, two fundamental concepts in chemistry and. in particle physics, the threshold energy for production of a particle. Difference Of Threshold Energy And Activation Energy.
From www.nuclear-power.com
Critical Energy Threshold Energy for Fission Difference Of Threshold Energy And Activation Energy activation energy is the minimum energy required for a chemical reaction to occur. learn the difference between activation energy and threshold energy, two fundamental concepts in chemistry and. Threshold energy is the minimum energy. Activation energy is the minimum energy required for reactants to collide. learn the difference between threshold energy and activation energy, two concepts in. Difference Of Threshold Energy And Activation Energy.
From thechemistrynotes.com
Activation Energy Definition, Unit, Formula, Calculations Difference Of Threshold Energy And Activation Energy Learn how activation energy relates. in particle physics, the threshold energy for production of a particle is the minimum kinetic energy a pair of traveling particles. — learn the difference between threshold energy and activation energy in chemical reactions. activation energy is the minimum energy required for a chemical reaction to occur. learn the difference between. Difference Of Threshold Energy And Activation Energy.
From dxorgzvns.blob.core.windows.net
Threshold Energy Of Nuclear Reaction at Lloyd Mueller blog Difference Of Threshold Energy And Activation Energy learn how to calculate activation energy and how it affects reaction rates. learn the difference between activation energy and threshold energy, two fundamental concepts in chemistry and. Learn how activation energy affects. — activation energy plays a crucial role in determining the rate of a chemical reaction; Activation energy is the minimum energy required for reactants to. Difference Of Threshold Energy And Activation Energy.
From chemistry.stackexchange.com
physical chemistry How exactly is activation energy defined Difference Of Threshold Energy And Activation Energy Learn how activation energy affects. learn the difference between activation energy and threshold energy, two fundamental concepts in chemistry and. activation energy is the minimum energy required for a chemical reaction to occur. — learn the difference between threshold energy and activation energy in chemical reactions. learn the difference between threshold energy and activation energy, two. Difference Of Threshold Energy And Activation Energy.
From byjus.com
Activation Energy Definition, Formula, SI Units, Examples, Calculation Difference Of Threshold Energy And Activation Energy Learn how activation energy affects. — activation energy plays a crucial role in determining the rate of a chemical reaction; Activation energy is the minimum energy required for reactants to collide. learn how to calculate activation energy and how it affects reaction rates. Threshold energy is the minimum energy. learn the difference between threshold energy and activation. Difference Of Threshold Energy And Activation Energy.
From socratic.org
What are activation energies? Socratic Difference Of Threshold Energy And Activation Energy activation energy is the minimum amount of energy that must be available to reactants for a chemical reaction to occur. learn the difference between activation energy and threshold energy, two fundamental concepts in chemistry and. Activation energy is the minimum energy required for reactants to collide. Threshold energy is the minimum energy. in particle physics, the threshold. Difference Of Threshold Energy And Activation Energy.
From www.youtube.com
Difference between Threshold energy and activation energy.Lecture 2 of Difference Of Threshold Energy And Activation Energy Activation energy is the minimum energy required for reactants to collide. Threshold energy is the minimum energy. — activation energy plays a crucial role in determining the rate of a chemical reaction; learn the difference between activation energy and threshold energy, two fundamental concepts in chemistry and. Learn how activation energy affects. in particle physics, the threshold. Difference Of Threshold Energy And Activation Energy.
From www.differencebetween.com
Difference Between Activation Energy and Threshold Energy Compare the Difference Of Threshold Energy And Activation Energy — learn the difference between threshold energy and activation energy in chemical reactions. Activation energy is the minimum energy required for reactants to collide. learn how to calculate activation energy and how it affects reaction rates. — activation energy plays a crucial role in determining the rate of a chemical reaction; Learn how activation energy relates. . Difference Of Threshold Energy And Activation Energy.
From www.slideserve.com
PPT Chemical PowerPoint Presentation, free download ID2428843 Difference Of Threshold Energy And Activation Energy Learn how activation energy relates. — activation energy plays a crucial role in determining the rate of a chemical reaction; — learn the difference between threshold energy and activation energy in chemical reactions. Activation energy is the minimum energy required for reactants to collide. activation energy is the minimum energy required for a chemical reaction to occur.. Difference Of Threshold Energy And Activation Energy.
From schematicrasariopm.z4.web.core.windows.net
Energy Diagram Activation Energy Difference Of Threshold Energy And Activation Energy learn the difference between threshold energy and activation energy, two concepts in chemical kinetics. activation energy is the minimum energy required for a chemical reaction to occur. — learn the difference between threshold energy and activation energy in chemical reactions. Learn how activation energy affects. Activation energy is the minimum energy required for reactants to collide. . Difference Of Threshold Energy And Activation Energy.