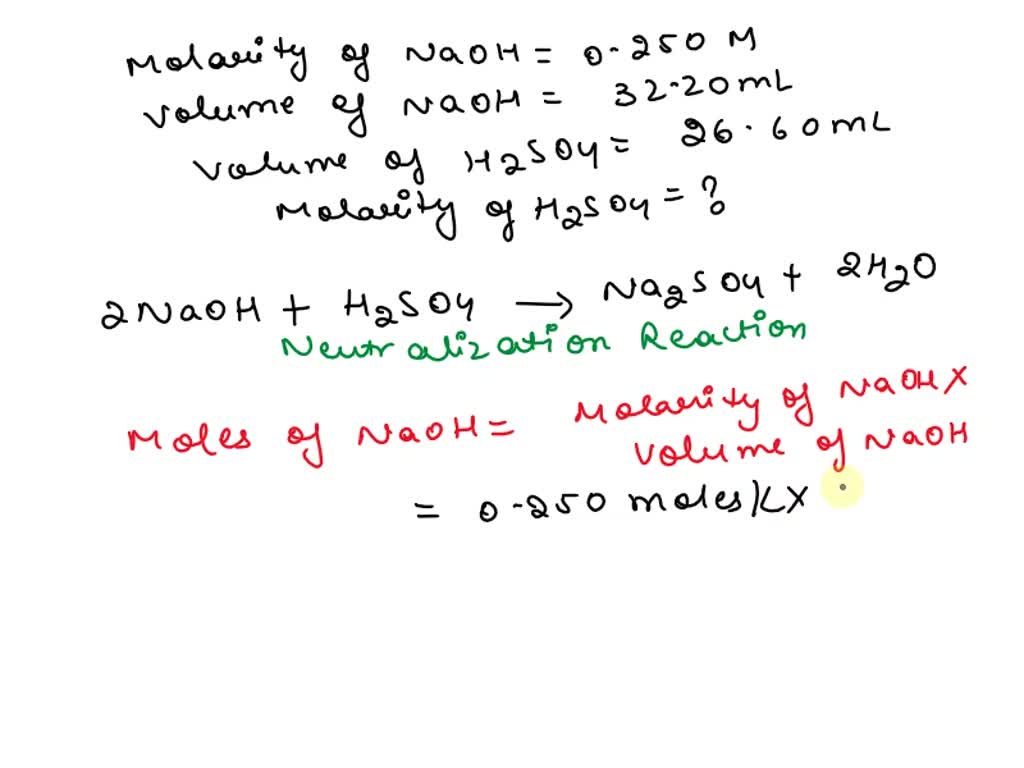Sulphuric Acid Titration Solution . The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide,. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. The sulphuric acid has an unknown concentration. A simple titration with sulfuric acid and sodium hydroxide is performed. Sulfuric acid is a strong diprotic. Sulfuric acid is a diprotic acid, with a pk a2 of 1.99 (the first k a value is very large and the acid dissociation reaction goes to completion, which. Then you will fill a burette with sodium. In this experiment, you will use a pipette to measure some sulphuric acid into a beaker. In my latest chem lab the objective was to create a primary standard of $\ce{naoh}$ and use it to determine the concentration of. First of all, as sulfuric acid is diprotic,. Determination of sulfuric acid concentration is very similar to titration of hydrochloric acid, although there are two important diferences.
from www.numerade.com
In this experiment, you will use a pipette to measure some sulphuric acid into a beaker. First of all, as sulfuric acid is diprotic,. In my latest chem lab the objective was to create a primary standard of $\ce{naoh}$ and use it to determine the concentration of. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide,. Then you will fill a burette with sodium. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. Determination of sulfuric acid concentration is very similar to titration of hydrochloric acid, although there are two important diferences. A simple titration with sulfuric acid and sodium hydroxide is performed. The sulphuric acid has an unknown concentration. Sulfuric acid is a strong diprotic.
SOLVED Q3 In a titration of sulfuric acid against sodium hydroxide
Sulphuric Acid Titration Solution Sulfuric acid is a strong diprotic. Sulfuric acid is a diprotic acid, with a pk a2 of 1.99 (the first k a value is very large and the acid dissociation reaction goes to completion, which. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. Determination of sulfuric acid concentration is very similar to titration of hydrochloric acid, although there are two important diferences. In my latest chem lab the objective was to create a primary standard of $\ce{naoh}$ and use it to determine the concentration of. Sulfuric acid is a strong diprotic. First of all, as sulfuric acid is diprotic,. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide,. Then you will fill a burette with sodium. In this experiment, you will use a pipette to measure some sulphuric acid into a beaker. A simple titration with sulfuric acid and sodium hydroxide is performed. The sulphuric acid has an unknown concentration.
From www.weiyel.com
Reference Materials Sulphuric Acid Titration Solution Sulfuric acid is a diprotic acid, with a pk a2 of 1.99 (the first k a value is very large and the acid dissociation reaction goes to completion, which. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. Then you will fill a burette with sodium. Determination of sulfuric. Sulphuric Acid Titration Solution.
From www.weiyel.com
Reference Materials Sulphuric Acid Titration Solution In this experiment, you will use a pipette to measure some sulphuric acid into a beaker. First of all, as sulfuric acid is diprotic,. Sulfuric acid is a diprotic acid, with a pk a2 of 1.99 (the first k a value is very large and the acid dissociation reaction goes to completion, which. A simple titration with sulfuric acid and. Sulphuric Acid Titration Solution.
From byjus.com
36. Assertion Sodium Carbonate can be titrated against sulphuric acid Sulphuric Acid Titration Solution Then you will fill a burette with sodium. In my latest chem lab the objective was to create a primary standard of $\ce{naoh}$ and use it to determine the concentration of. Sulfuric acid is a diprotic acid, with a pk a2 of 1.99 (the first k a value is very large and the acid dissociation reaction goes to completion, which.. Sulphuric Acid Titration Solution.
From brainly.com
A solution of sulfuric acid is titrated with a solution of sodium Sulphuric Acid Titration Solution First of all, as sulfuric acid is diprotic,. The sulphuric acid has an unknown concentration. Determination of sulfuric acid concentration is very similar to titration of hydrochloric acid, although there are two important diferences. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide,. Sulfuric acid is a diprotic acid,. Sulphuric Acid Titration Solution.
From mavink.com
Titration Reaction Sulphuric Acid Titration Solution Determination of sulfuric acid concentration is very similar to titration of hydrochloric acid, although there are two important diferences. First of all, as sulfuric acid is diprotic,. Sulfuric acid is a strong diprotic. Sulfuric acid is a diprotic acid, with a pk a2 of 1.99 (the first k a value is very large and the acid dissociation reaction goes to. Sulphuric Acid Titration Solution.
From www.myxxgirl.com
What Is Double Titration Theory By Using Sulphuric Acid My XXX Hot Girl Sulphuric Acid Titration Solution A simple titration with sulfuric acid and sodium hydroxide is performed. In my latest chem lab the objective was to create a primary standard of $\ce{naoh}$ and use it to determine the concentration of. Sulfuric acid is a strong diprotic. Determination of sulfuric acid concentration is very similar to titration of hydrochloric acid, although there are two important diferences. The. Sulphuric Acid Titration Solution.
From knowledge.carolina.com
Titrations Techniques and Calculations Carolina Knowledge Center Sulphuric Acid Titration Solution Then you will fill a burette with sodium. The sulphuric acid has an unknown concentration. Sulfuric acid is a diprotic acid, with a pk a2 of 1.99 (the first k a value is very large and the acid dissociation reaction goes to completion, which. First of all, as sulfuric acid is diprotic,. Sulfuric acid is a strong diprotic. The example. Sulphuric Acid Titration Solution.
From vdocuments.mx
Determining the End Point in the Titration of Sulphuric Acid and Sodium Sulphuric Acid Titration Solution Sulfuric acid is a strong diprotic. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide,. In my latest chem lab the objective was to create a primary standard of $\ce{naoh}$ and use it to determine the concentration of. First of all, as sulfuric acid is diprotic,. The example below. Sulphuric Acid Titration Solution.
From hxemhsffb.blob.core.windows.net
Sulphuric Acid Titration Experiment at Lillie Cunningham blog Sulphuric Acid Titration Solution In my latest chem lab the objective was to create a primary standard of $\ce{naoh}$ and use it to determine the concentration of. Sulfuric acid is a strong diprotic. Then you will fill a burette with sodium. In this experiment, you will use a pipette to measure some sulphuric acid into a beaker. First of all, as sulfuric acid is. Sulphuric Acid Titration Solution.
From studylib.net
2b. Ch 4 Barium hydroxide sulfuric acid titration Sulphuric Acid Titration Solution A simple titration with sulfuric acid and sodium hydroxide is performed. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide,. In my latest chem lab the objective was to create a primary standard of $\ce{naoh}$ and use it to determine the concentration of. In this experiment, you will use. Sulphuric Acid Titration Solution.
From www.chegg.com
Solved 2. 2. Student X performs an acidbase titration. He Sulphuric Acid Titration Solution The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. Then you will fill a burette with sodium. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide,. Determination of sulfuric acid concentration is very similar to titration of hydrochloric. Sulphuric Acid Titration Solution.
From www.coursehero.com
[Solved] A 25.00 ml volume of sulfuric acid was titrated with a Sulphuric Acid Titration Solution The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. Sulfuric acid is a diprotic acid, with a pk a2 of 1.99 (the first k a value is very large and the acid dissociation reaction goes to completion, which. Then you will fill a burette with sodium. Sulfuric acid is. Sulphuric Acid Titration Solution.
From shopee.ph
☃Dilute sulfuric acid chemical experiment titration standard solution 0 Sulphuric Acid Titration Solution First of all, as sulfuric acid is diprotic,. Determination of sulfuric acid concentration is very similar to titration of hydrochloric acid, although there are two important diferences. Sulfuric acid is a strong diprotic. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide,. Then you will fill a burette with. Sulphuric Acid Titration Solution.
From www.chegg.com
Solved 1. The titration of 25.00 mL of a sulfuric acid Sulphuric Acid Titration Solution Sulfuric acid is a diprotic acid, with a pk a2 of 1.99 (the first k a value is very large and the acid dissociation reaction goes to completion, which. In my latest chem lab the objective was to create a primary standard of $\ce{naoh}$ and use it to determine the concentration of. In this experiment, you will use a pipette. Sulphuric Acid Titration Solution.
From hxemhsffb.blob.core.windows.net
Sulphuric Acid Titration Experiment at Lillie Cunningham blog Sulphuric Acid Titration Solution In my latest chem lab the objective was to create a primary standard of $\ce{naoh}$ and use it to determine the concentration of. Sulfuric acid is a strong diprotic. Sulfuric acid is a diprotic acid, with a pk a2 of 1.99 (the first k a value is very large and the acid dissociation reaction goes to completion, which. The example. Sulphuric Acid Titration Solution.
From ar.inspiredpencil.com
Titration Setup Diagram Sulphuric Acid Titration Solution Sulfuric acid is a strong diprotic. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. Then you will fill a burette with sodium. First of all, as sulfuric acid is diprotic,. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with. Sulphuric Acid Titration Solution.
From www.sliderbase.com
Titration Sulphuric Acid Titration Solution The sulphuric acid has an unknown concentration. Sulfuric acid is a strong diprotic. Determination of sulfuric acid concentration is very similar to titration of hydrochloric acid, although there are two important diferences. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. In my latest chem lab the objective was. Sulphuric Acid Titration Solution.
From www.studypool.com
SOLUTION Titration to determine the concentration of a solution of Sulphuric Acid Titration Solution Then you will fill a burette with sodium. The sulphuric acid has an unknown concentration. First of all, as sulfuric acid is diprotic,. Determination of sulfuric acid concentration is very similar to titration of hydrochloric acid, although there are two important diferences. Sulfuric acid is a strong diprotic. A simple titration with sulfuric acid and sodium hydroxide is performed. The. Sulphuric Acid Titration Solution.
From norah-chapter.blogspot.com
Sodium Bicarbonate And Sulfuric Acid Equation 20+ Pages Explanation Doc Sulphuric Acid Titration Solution Sulfuric acid is a strong diprotic. Sulfuric acid is a diprotic acid, with a pk a2 of 1.99 (the first k a value is very large and the acid dissociation reaction goes to completion, which. In this experiment, you will use a pipette to measure some sulphuric acid into a beaker. In my latest chem lab the objective was to. Sulphuric Acid Titration Solution.
From www.chegg.com
Solved PART B Acid Base Titration Lab Report I Sulphuric Acid Titration Solution In my latest chem lab the objective was to create a primary standard of $\ce{naoh}$ and use it to determine the concentration of. Sulfuric acid is a strong diprotic. Then you will fill a burette with sodium. Sulfuric acid is a diprotic acid, with a pk a2 of 1.99 (the first k a value is very large and the acid. Sulphuric Acid Titration Solution.
From www.academia.edu
(DOC) To determine the concentration of sulfuric acid by titration Sulphuric Acid Titration Solution In my latest chem lab the objective was to create a primary standard of $\ce{naoh}$ and use it to determine the concentration of. Sulfuric acid is a strong diprotic. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide,. Sulfuric acid is a diprotic acid, with a pk a2 of. Sulphuric Acid Titration Solution.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Sulphuric Acid Titration Solution Sulfuric acid is a strong diprotic. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide,. Determination of sulfuric acid concentration is very similar to titration of hydrochloric acid, although there are two important diferences. In this experiment, you will use a pipette to measure some sulphuric acid into a. Sulphuric Acid Titration Solution.
From giomxxhve.blob.core.windows.net
Sulfuric Acid Titration Experiment at Frederick Duchesne blog Sulphuric Acid Titration Solution The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. The sulphuric acid has an unknown concentration. In this experiment, you will use a pipette to measure some sulphuric acid into a beaker. Sulfuric acid is a strong diprotic. A simple titration with sulfuric acid and sodium hydroxide is performed.. Sulphuric Acid Titration Solution.
From giomxxhve.blob.core.windows.net
Sulfuric Acid Titration Experiment at Frederick Duchesne blog Sulphuric Acid Titration Solution Sulfuric acid is a diprotic acid, with a pk a2 of 1.99 (the first k a value is very large and the acid dissociation reaction goes to completion, which. Then you will fill a burette with sodium. Sulfuric acid is a strong diprotic. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid. Sulphuric Acid Titration Solution.
From gbu-presnenskij.ru
Titration Of Sulfuric Acid And Sodium Hydroxide Science, 45 OFF Sulphuric Acid Titration Solution A simple titration with sulfuric acid and sodium hydroxide is performed. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide,. First of all, as sulfuric acid is diprotic,. In this experiment, you will use a pipette to measure some sulphuric acid into a beaker. Determination of sulfuric acid concentration. Sulphuric Acid Titration Solution.
From www.bartleby.com
Answered Q3 In a titration of sulfuric acid… bartleby Sulphuric Acid Titration Solution First of all, as sulfuric acid is diprotic,. A simple titration with sulfuric acid and sodium hydroxide is performed. In my latest chem lab the objective was to create a primary standard of $\ce{naoh}$ and use it to determine the concentration of. In this experiment, you will use a pipette to measure some sulphuric acid into a beaker. Sulfuric acid. Sulphuric Acid Titration Solution.
From www.numerade.com
SOLVED Experiment 22 Acids and Bases Analysis The titration of 25.00 Sulphuric Acid Titration Solution The sulphuric acid has an unknown concentration. In this experiment, you will use a pipette to measure some sulphuric acid into a beaker. Then you will fill a burette with sodium. In my latest chem lab the objective was to create a primary standard of $\ce{naoh}$ and use it to determine the concentration of. First of all, as sulfuric acid. Sulphuric Acid Titration Solution.
From syatillakmk.blogspot.com
SimplyChemistry TITRATION OF SULFURIC ACID WITH SODIUM HYDROXIDE Sulphuric Acid Titration Solution Determination of sulfuric acid concentration is very similar to titration of hydrochloric acid, although there are two important diferences. First of all, as sulfuric acid is diprotic,. Then you will fill a burette with sodium. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide,. A simple titration with sulfuric. Sulphuric Acid Titration Solution.
From www.aladdinsci.com
Sulfuric acid titration solution 1mol/L prefix CAS No. 7664939 Sulphuric Acid Titration Solution In this experiment, you will use a pipette to measure some sulphuric acid into a beaker. A simple titration with sulfuric acid and sodium hydroxide is performed. Determination of sulfuric acid concentration is very similar to titration of hydrochloric acid, although there are two important diferences. The sulphuric acid has an unknown concentration. First of all, as sulfuric acid is. Sulphuric Acid Titration Solution.
From www.numerade.com
SOLVED Q3 In a titration of sulfuric acid against sodium hydroxide Sulphuric Acid Titration Solution Determination of sulfuric acid concentration is very similar to titration of hydrochloric acid, although there are two important diferences. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. A simple titration with sulfuric acid and sodium hydroxide is performed. The example below demonstrates the technique to solve a titration. Sulphuric Acid Titration Solution.
From www.studypool.com
SOLUTION Titration to determine the concentration of a solution of Sulphuric Acid Titration Solution Determination of sulfuric acid concentration is very similar to titration of hydrochloric acid, although there are two important diferences. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. In this experiment, you will use a pipette to measure some sulphuric acid into a beaker. Sulfuric acid is a strong. Sulphuric Acid Titration Solution.
From www.youtube.com
Acid Base Titration Sulfuric acid and Sodium hydroxide YouTube Sulphuric Acid Titration Solution In this experiment, you will use a pipette to measure some sulphuric acid into a beaker. The sulphuric acid has an unknown concentration. Sulfuric acid is a diprotic acid, with a pk a2 of 1.99 (the first k a value is very large and the acid dissociation reaction goes to completion, which. The example below demonstrates the technique to solve. Sulphuric Acid Titration Solution.
From www.electro-glo.com
Sulfuric Acid Titrating Solution N/10 (0.100N) (1 ml = 100 ppm CaCO3/50 Sulphuric Acid Titration Solution First of all, as sulfuric acid is diprotic,. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide,. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. In my latest chem lab the objective was to create a primary. Sulphuric Acid Titration Solution.
From www.pchemlabs.com
Titrating Solution 460S0226 SOLN N/50 Sulfuric Acid Titrant, Form Sulphuric Acid Titration Solution First of all, as sulfuric acid is diprotic,. Determination of sulfuric acid concentration is very similar to titration of hydrochloric acid, although there are two important diferences. Then you will fill a burette with sodium. A simple titration with sulfuric acid and sodium hydroxide is performed. The example below demonstrates the technique to solve a titration problem for a titration. Sulphuric Acid Titration Solution.
From www.youtube.com
Titration of Sulfuric Acid YouTube Sulphuric Acid Titration Solution The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. The sulphuric acid has an unknown concentration. Sulfuric acid is a strong diprotic. Determination of sulfuric acid concentration is very similar to titration of hydrochloric acid, although there are two important diferences. Sulfuric acid is a diprotic acid, with a. Sulphuric Acid Titration Solution.