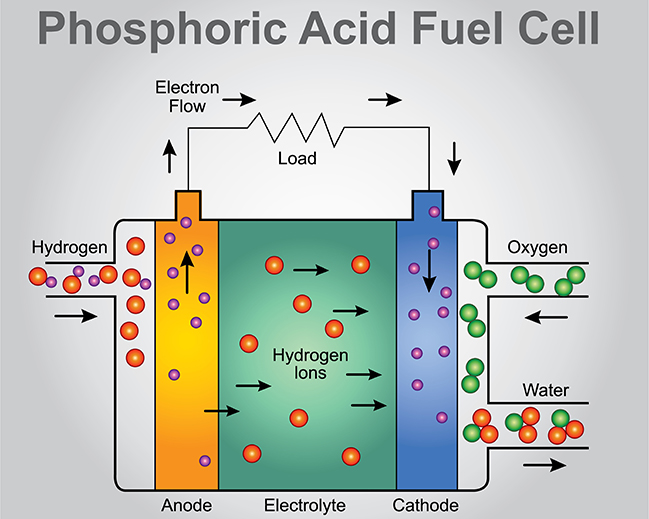
Fuel Cell Electrode Reactions . It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical reactions. Revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the chemistry experts at. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. Oxygen is supplied to a similar electrode except that the catalyst is. Learn types of fuel cell, working and more here. In contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. What is a fuel cell? In this section, we describe the chemistry behind. Hydrogen + oxygen → water.
from www.denora.com
A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. Learn types of fuel cell, working and more here. Revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the chemistry experts at. It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical reactions. Oxygen is supplied to a similar electrode except that the catalyst is. What is a fuel cell? In contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. In this section, we describe the chemistry behind. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. Hydrogen + oxygen → water.
Anodes and Cathodes for Fuel Cells De Nora Electrode and Water
Fuel Cell Electrode Reactions A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. In contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. In this section, we describe the chemistry behind. Revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the chemistry experts at. What is a fuel cell? Oxygen is supplied to a similar electrode except that the catalyst is. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical reactions. Learn types of fuel cell, working and more here. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. Hydrogen + oxygen → water.
From www.researchgate.net
Oxidation reactions in fuel cells (cathodic and anodic half reactions Fuel Cell Electrode Reactions Hydrogen + oxygen → water. Oxygen is supplied to a similar electrode except that the catalyst is. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical reactions. Revision notes on. Fuel Cell Electrode Reactions.
From mrselliott.wordpress.com
Electrochemistry, featuring electrolysis and fuel cells. mrs elliott Fuel Cell Electrode Reactions It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical reactions. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. Oxygen is supplied to a similar electrode except that the catalyst is. Revision notes on electrode reactions in hydrogen fuel. Fuel Cell Electrode Reactions.
From www.youtube.com
Fuel Cell (0601) Thermodynamics Electrochem Reaction YouTube Fuel Cell Electrode Reactions Oxygen is supplied to a similar electrode except that the catalyst is. In contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. What is a fuel cell? Hydrogen + oxygen → water. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst.. Fuel Cell Electrode Reactions.
From large.stanford.edu
Hydrogen Fuel Cells Fuel Cell Electrode Reactions What is a fuel cell? Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. In this section, we describe the chemistry behind. Hydrogen + oxygen → water. In contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. It is defined as. Fuel Cell Electrode Reactions.
From chem.libretexts.org
20.4 Cell Potential Under Standard Conditions Chemistry LibreTexts Fuel Cell Electrode Reactions A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. In this section, we describe the chemistry behind. Hydrogen + oxygen → water. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. Revision notes on. Fuel Cell Electrode Reactions.
From www.researchgate.net
Schematic representation of a UE cell showing the electrode reactions Fuel Cell Electrode Reactions In this section, we describe the chemistry behind. Learn types of fuel cell, working and more here. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. Revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the chemistry. Fuel Cell Electrode Reactions.
From www.slideserve.com
PPT Fuel Cell Catalysts Based on Metal Nanoparticles PowerPoint Fuel Cell Electrode Reactions Learn types of fuel cell, working and more here. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. It is defined as an electrochemical cell. Fuel Cell Electrode Reactions.
From www.researchgate.net
Schematics of a) Alkaline Fuel Cell (AFC), b) Phosphoric Acid Fuel Cell Fuel Cell Electrode Reactions Hydrogen + oxygen → water. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. Oxygen is supplied to a similar electrode except that the catalyst is. Learn types of fuel cell, working and more here. Hydrogen enters the cell through a porous carbon electrode which. Fuel Cell Electrode Reactions.
From www.researchgate.net
Electrochemical reactions in a fuel cell with (A) H 2 O 2 and (B) CH 4 Fuel Cell Electrode Reactions Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. In this section, we describe the chemistry behind. A fuel cell consists of two electrodes—a negative electrode (or anode) and a. Fuel Cell Electrode Reactions.
From www.preprints.org
Educational Model of Electric Potential, Electrochemical Reactions, and Fuel Cell Electrode Reactions In contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. Revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the chemistry experts at. Hydrogen + oxygen → water. Learn types of fuel cell, working and more here. Oxygen is. Fuel Cell Electrode Reactions.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Fuel Cell Electrode Reactions Hydrogen + oxygen → water. What is a fuel cell? In contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. Revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the chemistry experts at. A fuel cell is an electrochemical. Fuel Cell Electrode Reactions.
From www.scribd.com
An InDepth Explanation of How Fuel Cells Generate Electricity Through Fuel Cell Electrode Reactions Revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the chemistry experts at. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. In contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more. Fuel Cell Electrode Reactions.
From www.electroniclinic.com
Hydrogen Fuel Cell, Application of Fuel Cells, construction, and Working Fuel Cell Electrode Reactions In this section, we describe the chemistry behind. It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical reactions. Hydrogen + oxygen → water. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. In contrast, a fuel cell is a. Fuel Cell Electrode Reactions.
From www.researchgate.net
15 Fuel cell types and reactions. Download Scientific Diagram Fuel Cell Electrode Reactions What is a fuel cell? Revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the chemistry experts at. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. A fuel cell is an electrochemical cell in which a fuel donates electrons at. Fuel Cell Electrode Reactions.
From www.researchgate.net
Electrochemical reaction of PEM fuel cell electrode (cathode side) to Fuel Cell Electrode Reactions Hydrogen + oxygen → water. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. Oxygen is supplied to a similar electrode except that the catalyst is. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. In this section, we. Fuel Cell Electrode Reactions.
From www.cambridge.org
Multiscale imaging and transport modeling for fuel cell electrodes Fuel Cell Electrode Reactions Oxygen is supplied to a similar electrode except that the catalyst is. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. Hydrogen + oxygen → water. What is a fuel. Fuel Cell Electrode Reactions.
From www.researchgate.net
Layer structure and electrochemical reactions of PEM fuel cell Fuel Cell Electrode Reactions Oxygen is supplied to a similar electrode except that the catalyst is. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical reactions. In this section, we describe the chemistry behind. Hydrogen enters the cell. Fuel Cell Electrode Reactions.
From www.denora.com
Anodes and Cathodes for Fuel Cells De Nora Electrode and Water Fuel Cell Electrode Reactions A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. Learn types of fuel cell, working and more here. Oxygen is supplied to a similar electrode except that the catalyst is. It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical. Fuel Cell Electrode Reactions.
From www.researchgate.net
Schematic of a microbial fuel cell (a), microbial electrolysis cell Fuel Cell Electrode Reactions Revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the chemistry experts at. What is a fuel cell? Hydrogen + oxygen → water. Learn types of fuel cell, working and more here. Oxygen is supplied to a similar electrode except that the catalyst is. It is defined as an electrochemical cell that. Fuel Cell Electrode Reactions.
From www.researchgate.net
Design of a hybrid protonexchange membrane fuel cell Schematic of a Fuel Cell Electrode Reactions Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. What is a fuel cell? Revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the chemistry experts at. In contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more. Fuel Cell Electrode Reactions.
From www.nagwa.com
Question Video Identifying Which Equation Shows the Reaction at a Fuel Cell Electrode Reactions A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. In contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. Revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by. Fuel Cell Electrode Reactions.
From www.chegg.com
Solved Write the balanced halfreaction that occurs at the Fuel Cell Electrode Reactions Hydrogen + oxygen → water. In contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. In this section, we describe the chemistry behind. It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical reactions. A fuel cell consists of two electrodes—a negative. Fuel Cell Electrode Reactions.
From www.researchgate.net
Schematics of (a) experimental setup and electrode reactions in a Fuel Cell Electrode Reactions It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical reactions. Learn types of fuel cell, working and more here. In contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. Hydrogen enters the cell through a porous carbon electrode which also contains. Fuel Cell Electrode Reactions.
From www.zigya.com
What are fuel cells? Write the electrode reactions of a fuel cell Fuel Cell Electrode Reactions Revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the chemistry experts at. In this section, we describe the chemistry behind. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. Learn types of fuel cell, working and. Fuel Cell Electrode Reactions.
From www.mdpi.com
Energies Free FullText Principles and Materials Aspects of Direct Fuel Cell Electrode Reactions Learn types of fuel cell, working and more here. Revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the chemistry experts at. It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical reactions. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum. Fuel Cell Electrode Reactions.
From www.researchgate.net
Electrochemical reaction of PEM fuel cell electrode (cathode side) to Fuel Cell Electrode Reactions Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. What is a fuel cell? Oxygen is supplied to a similar electrode except that the catalyst is. Learn types of fuel cell, working and more here. Revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the chemistry. Fuel Cell Electrode Reactions.
From chemistry.stackexchange.com
electrochemistry Electrode polarity in fuel cells Chemistry Stack Fuel Cell Electrode Reactions In contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. Oxygen is supplied to a similar electrode except that the catalyst is. Revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the chemistry experts at. A fuel cell is. Fuel Cell Electrode Reactions.
From www.dreamstime.com
Hydrogen Fuel Cell Electrode Reactions Stock Photo Image of Fuel Cell Electrode Reactions Hydrogen + oxygen → water. Learn types of fuel cell, working and more here. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. In this section, we describe the chemistry behind. In contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or. Fuel Cell Electrode Reactions.
From www.researchgate.net
Schematic illustration of the double chamber microbial fuel cell Fuel Cell Electrode Reactions Hydrogen + oxygen → water. Learn types of fuel cell, working and more here. What is a fuel cell? Revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the chemistry experts at. In this section, we describe the chemistry behind. Oxygen is supplied to a similar electrode except that the catalyst is.. Fuel Cell Electrode Reactions.
From www.researchgate.net
Schematics of a) Alkaline Fuel Cell (AFC), b) Phosphoric Acid Fuel Cell Fuel Cell Electrode Reactions Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. In this section, we describe the chemistry behind. In contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. Oxygen is supplied to a similar electrode except that the catalyst is. A fuel. Fuel Cell Electrode Reactions.
From pubs.rsc.org
Trends in electrode development for next generation solid oxide fuel Fuel Cell Electrode Reactions In this section, we describe the chemistry behind. In contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. What is a fuel cell? A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. Hydrogen enters the cell. Fuel Cell Electrode Reactions.
From www.chfca.ca
About Fuel Cells CHFCA Fuel Cell Electrode Reactions Learn types of fuel cell, working and more here. Hydrogen + oxygen → water. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. What is a fuel cell? Revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the chemistry experts at.. Fuel Cell Electrode Reactions.
From studylib.net
Electrochemical Reactions in Polymer Electrolyte Fuel Cells Fuel Cell Electrode Reactions A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. What is a fuel cell? In contrast, a fuel cell is a galvanic cell that requires. Fuel Cell Electrode Reactions.
From www.researchgate.net
Schematics of a) Alkaline Fuel Cell (AFC), b) Phosphoric Acid Fuel Cell Fuel Cell Electrode Reactions Learn types of fuel cell, working and more here. Revision notes on electrode reactions in hydrogen fuel cells for the aqa gcse chemistry syllabus, written by the chemistry experts at. A fuel cell is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at the other electrode. Hydrogen enters the cell through a. Fuel Cell Electrode Reactions.
From www.betase.nl
Electrochemistry of the fuel cell Betase BV Fuel Cell Electrode Reactions In contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. Hydrogen + oxygen → water. What is a fuel cell? Oxygen is supplied to a similar electrode except that the catalyst is. It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical. Fuel Cell Electrode Reactions.