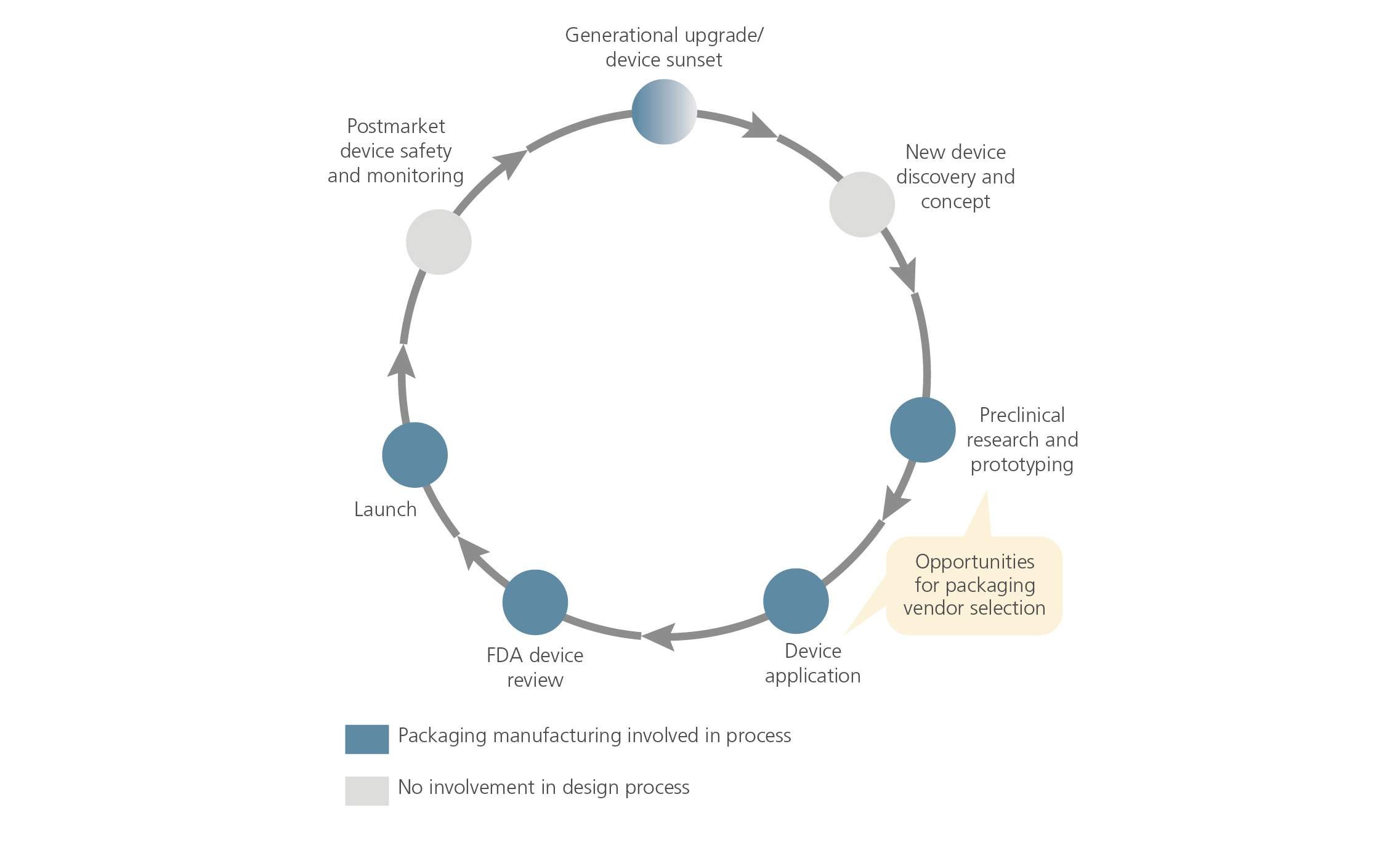
Medical Device Lifetime Definition . Let’s take a look at the definition of expected device lifetime. However, the definition of the device’s lifetime and shelf life ensures that users and patients are informed of the device’s achievable safety and. In prior guidance from the international medical device regulators forum (imdrf) 4, of essential principles of safety and performance of. The eu mdr/ivdr does not explicitly define shelf life, expiration date, expected lifetime/useful life, service life, or life cycle for medical devices. The guidance recommends documenting the defined lifetime. Iso 13485:2003, clause 7.1.3 offers advice on setting the lifetime of a device. Defining the 'expected device lifetime' of a medical device. · clearly define device lifetime in documentation and instructions, based on rigorous testing and risk management. Documenting lifetime claims & other sources of lifetime. Characteristics affecting lifetime and risk management. “it is a period during which the device is.
from mavink.com
Let’s take a look at the definition of expected device lifetime. In prior guidance from the international medical device regulators forum (imdrf) 4, of essential principles of safety and performance of. · clearly define device lifetime in documentation and instructions, based on rigorous testing and risk management. Documenting lifetime claims & other sources of lifetime. The guidance recommends documenting the defined lifetime. Defining the 'expected device lifetime' of a medical device. However, the definition of the device’s lifetime and shelf life ensures that users and patients are informed of the device’s achievable safety and. The eu mdr/ivdr does not explicitly define shelf life, expiration date, expected lifetime/useful life, service life, or life cycle for medical devices. Iso 13485:2003, clause 7.1.3 offers advice on setting the lifetime of a device. Characteristics affecting lifetime and risk management.
Medical Device Life Cycle Phases
Medical Device Lifetime Definition The eu mdr/ivdr does not explicitly define shelf life, expiration date, expected lifetime/useful life, service life, or life cycle for medical devices. The eu mdr/ivdr does not explicitly define shelf life, expiration date, expected lifetime/useful life, service life, or life cycle for medical devices. Documenting lifetime claims & other sources of lifetime. Defining the 'expected device lifetime' of a medical device. · clearly define device lifetime in documentation and instructions, based on rigorous testing and risk management. In prior guidance from the international medical device regulators forum (imdrf) 4, of essential principles of safety and performance of. “it is a period during which the device is. However, the definition of the device’s lifetime and shelf life ensures that users and patients are informed of the device’s achievable safety and. The guidance recommends documenting the defined lifetime. Iso 13485:2003, clause 7.1.3 offers advice on setting the lifetime of a device. Let’s take a look at the definition of expected device lifetime. Characteristics affecting lifetime and risk management.
From www.drugwatch.com
Overview of the Safe Medical Devices Act of 1990 Medical Device Lifetime Definition Characteristics affecting lifetime and risk management. “it is a period during which the device is. · clearly define device lifetime in documentation and instructions, based on rigorous testing and risk management. The guidance recommends documenting the defined lifetime. Documenting lifetime claims & other sources of lifetime. Defining the 'expected device lifetime' of a medical device. Let’s take a look at. Medical Device Lifetime Definition.
From laegemiddelstyrelsen.dk
Development of medical devices Medical Device Lifetime Definition The guidance recommends documenting the defined lifetime. Documenting lifetime claims & other sources of lifetime. Defining the 'expected device lifetime' of a medical device. Iso 13485:2003, clause 7.1.3 offers advice on setting the lifetime of a device. Characteristics affecting lifetime and risk management. In prior guidance from the international medical device regulators forum (imdrf) 4, of essential principles of safety. Medical Device Lifetime Definition.
From mdrsllc.com
Device Development Life Cycle Medical Device Lifetime Definition The eu mdr/ivdr does not explicitly define shelf life, expiration date, expected lifetime/useful life, service life, or life cycle for medical devices. “it is a period during which the device is. Defining the 'expected device lifetime' of a medical device. Documenting lifetime claims & other sources of lifetime. Iso 13485:2003, clause 7.1.3 offers advice on setting the lifetime of a. Medical Device Lifetime Definition.
From mavink.com
Medical Device Life Cycle Phases Medical Device Lifetime Definition In prior guidance from the international medical device regulators forum (imdrf) 4, of essential principles of safety and performance of. “it is a period during which the device is. Iso 13485:2003, clause 7.1.3 offers advice on setting the lifetime of a device. Let’s take a look at the definition of expected device lifetime. Defining the 'expected device lifetime' of a. Medical Device Lifetime Definition.
From www.slideserve.com
PPT Life cycle of medical devices PowerPoint Presentation, free Medical Device Lifetime Definition Let’s take a look at the definition of expected device lifetime. Defining the 'expected device lifetime' of a medical device. Characteristics affecting lifetime and risk management. The guidance recommends documenting the defined lifetime. In prior guidance from the international medical device regulators forum (imdrf) 4, of essential principles of safety and performance of. Documenting lifetime claims & other sources of. Medical Device Lifetime Definition.
From mavink.com
Medical Device Life Cycle Phases Medical Device Lifetime Definition Let’s take a look at the definition of expected device lifetime. Characteristics affecting lifetime and risk management. The eu mdr/ivdr does not explicitly define shelf life, expiration date, expected lifetime/useful life, service life, or life cycle for medical devices. Iso 13485:2003, clause 7.1.3 offers advice on setting the lifetime of a device. Documenting lifetime claims & other sources of lifetime.. Medical Device Lifetime Definition.
From 4easyreg.com
Medical Device Lifetime An Overview 4EasyReg Medical Device Lifetime Definition · clearly define device lifetime in documentation and instructions, based on rigorous testing and risk management. However, the definition of the device’s lifetime and shelf life ensures that users and patients are informed of the device’s achievable safety and. “it is a period during which the device is. The guidance recommends documenting the defined lifetime. In prior guidance from the. Medical Device Lifetime Definition.
From mavink.com
Medical Device Life Cycle Phases Medical Device Lifetime Definition However, the definition of the device’s lifetime and shelf life ensures that users and patients are informed of the device’s achievable safety and. Let’s take a look at the definition of expected device lifetime. Characteristics affecting lifetime and risk management. In prior guidance from the international medical device regulators forum (imdrf) 4, of essential principles of safety and performance of.. Medical Device Lifetime Definition.
From www.linkedin.com
Navigating the Lifespan of Medical Devices Unveiling the calculation Medical Device Lifetime Definition Characteristics affecting lifetime and risk management. The eu mdr/ivdr does not explicitly define shelf life, expiration date, expected lifetime/useful life, service life, or life cycle for medical devices. However, the definition of the device’s lifetime and shelf life ensures that users and patients are informed of the device’s achievable safety and. Documenting lifetime claims & other sources of lifetime. “it. Medical Device Lifetime Definition.
From www.slideserve.com
PPT Life cycle of medical devices PowerPoint Presentation ID254282 Medical Device Lifetime Definition Characteristics affecting lifetime and risk management. Let’s take a look at the definition of expected device lifetime. In prior guidance from the international medical device regulators forum (imdrf) 4, of essential principles of safety and performance of. “it is a period during which the device is. · clearly define device lifetime in documentation and instructions, based on rigorous testing and. Medical Device Lifetime Definition.
From www.greenlight.guru
Medical Device Clinical Trials An Overview [+Types Explained] Medical Device Lifetime Definition The guidance recommends documenting the defined lifetime. However, the definition of the device’s lifetime and shelf life ensures that users and patients are informed of the device’s achievable safety and. The eu mdr/ivdr does not explicitly define shelf life, expiration date, expected lifetime/useful life, service life, or life cycle for medical devices. Let’s take a look at the definition of. Medical Device Lifetime Definition.
From www.researchgate.net
Medical device lifecycle management (see online version for colours Medical Device Lifetime Definition Iso 13485:2003, clause 7.1.3 offers advice on setting the lifetime of a device. The guidance recommends documenting the defined lifetime. “it is a period during which the device is. Defining the 'expected device lifetime' of a medical device. The eu mdr/ivdr does not explicitly define shelf life, expiration date, expected lifetime/useful life, service life, or life cycle for medical devices.. Medical Device Lifetime Definition.
From aganamed.com
Educational Literature AganaMed LLC Medical Device Lifetime Definition Iso 13485:2003, clause 7.1.3 offers advice on setting the lifetime of a device. The guidance recommends documenting the defined lifetime. Documenting lifetime claims & other sources of lifetime. However, the definition of the device’s lifetime and shelf life ensures that users and patients are informed of the device’s achievable safety and. “it is a period during which the device is.. Medical Device Lifetime Definition.
From www.jli.edu.in
5 Phases of Medical Device Development Process JLI Blog Medical Device Lifetime Definition Defining the 'expected device lifetime' of a medical device. However, the definition of the device’s lifetime and shelf life ensures that users and patients are informed of the device’s achievable safety and. Characteristics affecting lifetime and risk management. In prior guidance from the international medical device regulators forum (imdrf) 4, of essential principles of safety and performance of. Let’s take. Medical Device Lifetime Definition.
From www.vrogue.co
The Process Phases Of Medical Device Development Down vrogue.co Medical Device Lifetime Definition The eu mdr/ivdr does not explicitly define shelf life, expiration date, expected lifetime/useful life, service life, or life cycle for medical devices. · clearly define device lifetime in documentation and instructions, based on rigorous testing and risk management. “it is a period during which the device is. Characteristics affecting lifetime and risk management. However, the definition of the device’s lifetime. Medical Device Lifetime Definition.
From www.researchgate.net
The LifeCycle of a Medical Device. Download Scientific Diagram Medical Device Lifetime Definition However, the definition of the device’s lifetime and shelf life ensures that users and patients are informed of the device’s achievable safety and. The eu mdr/ivdr does not explicitly define shelf life, expiration date, expected lifetime/useful life, service life, or life cycle for medical devices. Let’s take a look at the definition of expected device lifetime. In prior guidance from. Medical Device Lifetime Definition.
From medicaldevicehq.com
An illustrated guide to medical device software development Medical Device Lifetime Definition Defining the 'expected device lifetime' of a medical device. The eu mdr/ivdr does not explicitly define shelf life, expiration date, expected lifetime/useful life, service life, or life cycle for medical devices. Iso 13485:2003, clause 7.1.3 offers advice on setting the lifetime of a device. “it is a period during which the device is. The guidance recommends documenting the defined lifetime.. Medical Device Lifetime Definition.
From 4easyreg.com
Medical Device Lifetime An Overview 4EasyReg Medical Device Lifetime Definition Characteristics affecting lifetime and risk management. The eu mdr/ivdr does not explicitly define shelf life, expiration date, expected lifetime/useful life, service life, or life cycle for medical devices. However, the definition of the device’s lifetime and shelf life ensures that users and patients are informed of the device’s achievable safety and. Defining the 'expected device lifetime' of a medical device.. Medical Device Lifetime Definition.
From www.vrogue.co
Understanding The 7 Phases Of Medical Device Developm vrogue.co Medical Device Lifetime Definition The guidance recommends documenting the defined lifetime. Let’s take a look at the definition of expected device lifetime. Characteristics affecting lifetime and risk management. Defining the 'expected device lifetime' of a medical device. “it is a period during which the device is. Documenting lifetime claims & other sources of lifetime. The eu mdr/ivdr does not explicitly define shelf life, expiration. Medical Device Lifetime Definition.
From www.linkedin.com
What is the Lifetime of a Medical Device? Medical Device Lifetime Definition “it is a period during which the device is. The eu mdr/ivdr does not explicitly define shelf life, expiration date, expected lifetime/useful life, service life, or life cycle for medical devices. In prior guidance from the international medical device regulators forum (imdrf) 4, of essential principles of safety and performance of. · clearly define device lifetime in documentation and instructions,. Medical Device Lifetime Definition.
From www.i3cglobal.com
Medical Device Lifetime and Shelf Life I3CGLOBAL Medical Device Lifetime Definition The eu mdr/ivdr does not explicitly define shelf life, expiration date, expected lifetime/useful life, service life, or life cycle for medical devices. Iso 13485:2003, clause 7.1.3 offers advice on setting the lifetime of a device. In prior guidance from the international medical device regulators forum (imdrf) 4, of essential principles of safety and performance of. Characteristics affecting lifetime and risk. Medical Device Lifetime Definition.
From www.mdpi.com
Nanomaterials Free FullText Evolution of Wearable Devices with Medical Device Lifetime Definition However, the definition of the device’s lifetime and shelf life ensures that users and patients are informed of the device’s achievable safety and. · clearly define device lifetime in documentation and instructions, based on rigorous testing and risk management. Defining the 'expected device lifetime' of a medical device. “it is a period during which the device is. Characteristics affecting lifetime. Medical Device Lifetime Definition.
From www.slideserve.com
PPT Life cycle of medical devices PowerPoint Presentation ID254282 Medical Device Lifetime Definition However, the definition of the device’s lifetime and shelf life ensures that users and patients are informed of the device’s achievable safety and. The guidance recommends documenting the defined lifetime. “it is a period during which the device is. Defining the 'expected device lifetime' of a medical device. In prior guidance from the international medical device regulators forum (imdrf) 4,. Medical Device Lifetime Definition.
From english.igj.nl
Our supervision of medical technology Medical Technology Health and Medical Device Lifetime Definition “it is a period during which the device is. · clearly define device lifetime in documentation and instructions, based on rigorous testing and risk management. The eu mdr/ivdr does not explicitly define shelf life, expiration date, expected lifetime/useful life, service life, or life cycle for medical devices. Let’s take a look at the definition of expected device lifetime. Characteristics affecting. Medical Device Lifetime Definition.
From www.slideserve.com
PPT Crossing the Threshold ( FDA Regulatory Requirements for Medical Medical Device Lifetime Definition However, the definition of the device’s lifetime and shelf life ensures that users and patients are informed of the device’s achievable safety and. In prior guidance from the international medical device regulators forum (imdrf) 4, of essential principles of safety and performance of. Defining the 'expected device lifetime' of a medical device. Let’s take a look at the definition of. Medical Device Lifetime Definition.
From www.ni.com
Easing the Medical Device Life Cycle with Virtual Instrumentation Medical Device Lifetime Definition Let’s take a look at the definition of expected device lifetime. The eu mdr/ivdr does not explicitly define shelf life, expiration date, expected lifetime/useful life, service life, or life cycle for medical devices. Documenting lifetime claims & other sources of lifetime. However, the definition of the device’s lifetime and shelf life ensures that users and patients are informed of the. Medical Device Lifetime Definition.
From www.vrogue.co
Life Cycle Of A Medical Device Custom University Pape vrogue.co Medical Device Lifetime Definition “it is a period during which the device is. In prior guidance from the international medical device regulators forum (imdrf) 4, of essential principles of safety and performance of. Let’s take a look at the definition of expected device lifetime. Characteristics affecting lifetime and risk management. The guidance recommends documenting the defined lifetime. Iso 13485:2003, clause 7.1.3 offers advice on. Medical Device Lifetime Definition.
From www.vrogue.co
The 12 Phases Of Medical Device Development Meridian vrogue.co Medical Device Lifetime Definition The guidance recommends documenting the defined lifetime. Documenting lifetime claims & other sources of lifetime. Iso 13485:2003, clause 7.1.3 offers advice on setting the lifetime of a device. · clearly define device lifetime in documentation and instructions, based on rigorous testing and risk management. Characteristics affecting lifetime and risk management. However, the definition of the device’s lifetime and shelf life. Medical Device Lifetime Definition.
From www.mediconvillage.se
Medical Device Product Life Cycle Make compliance your success factor Medical Device Lifetime Definition · clearly define device lifetime in documentation and instructions, based on rigorous testing and risk management. The guidance recommends documenting the defined lifetime. Let’s take a look at the definition of expected device lifetime. In prior guidance from the international medical device regulators forum (imdrf) 4, of essential principles of safety and performance of. However, the definition of the device’s. Medical Device Lifetime Definition.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Medical Device Lifetime Definition The guidance recommends documenting the defined lifetime. Let’s take a look at the definition of expected device lifetime. The eu mdr/ivdr does not explicitly define shelf life, expiration date, expected lifetime/useful life, service life, or life cycle for medical devices. Documenting lifetime claims & other sources of lifetime. Characteristics affecting lifetime and risk management. Iso 13485:2003, clause 7.1.3 offers advice. Medical Device Lifetime Definition.
From operonstrategist.com
5 Phases Of Medical Device Development (Step By Step Process) Operon Medical Device Lifetime Definition Iso 13485:2003, clause 7.1.3 offers advice on setting the lifetime of a device. Defining the 'expected device lifetime' of a medical device. “it is a period during which the device is. The eu mdr/ivdr does not explicitly define shelf life, expiration date, expected lifetime/useful life, service life, or life cycle for medical devices. Let’s take a look at the definition. Medical Device Lifetime Definition.
From mavink.com
Examples Of Medical Devices Medical Device Lifetime Definition · clearly define device lifetime in documentation and instructions, based on rigorous testing and risk management. Documenting lifetime claims & other sources of lifetime. In prior guidance from the international medical device regulators forum (imdrf) 4, of essential principles of safety and performance of. Characteristics affecting lifetime and risk management. The guidance recommends documenting the defined lifetime. “it is a. Medical Device Lifetime Definition.
From www.bsigroup.com
Medical Device Lifetime BSI Medical Device Lifetime Definition The guidance recommends documenting the defined lifetime. Defining the 'expected device lifetime' of a medical device. Documenting lifetime claims & other sources of lifetime. Iso 13485:2003, clause 7.1.3 offers advice on setting the lifetime of a device. The eu mdr/ivdr does not explicitly define shelf life, expiration date, expected lifetime/useful life, service life, or life cycle for medical devices. In. Medical Device Lifetime Definition.
From operonstrategist.com
Software as a Medical Device (SaMD) IEC 62304 Certification Medical Device Lifetime Definition Defining the 'expected device lifetime' of a medical device. The eu mdr/ivdr does not explicitly define shelf life, expiration date, expected lifetime/useful life, service life, or life cycle for medical devices. The guidance recommends documenting the defined lifetime. · clearly define device lifetime in documentation and instructions, based on rigorous testing and risk management. “it is a period during which. Medical Device Lifetime Definition.
From qbdgroup.com
MEDICAL DEVICE GUIDE The Pathway from Idea to Patient QbD Group Medical Device Lifetime Definition Characteristics affecting lifetime and risk management. Let’s take a look at the definition of expected device lifetime. · clearly define device lifetime in documentation and instructions, based on rigorous testing and risk management. “it is a period during which the device is. The eu mdr/ivdr does not explicitly define shelf life, expiration date, expected lifetime/useful life, service life, or life. Medical Device Lifetime Definition.