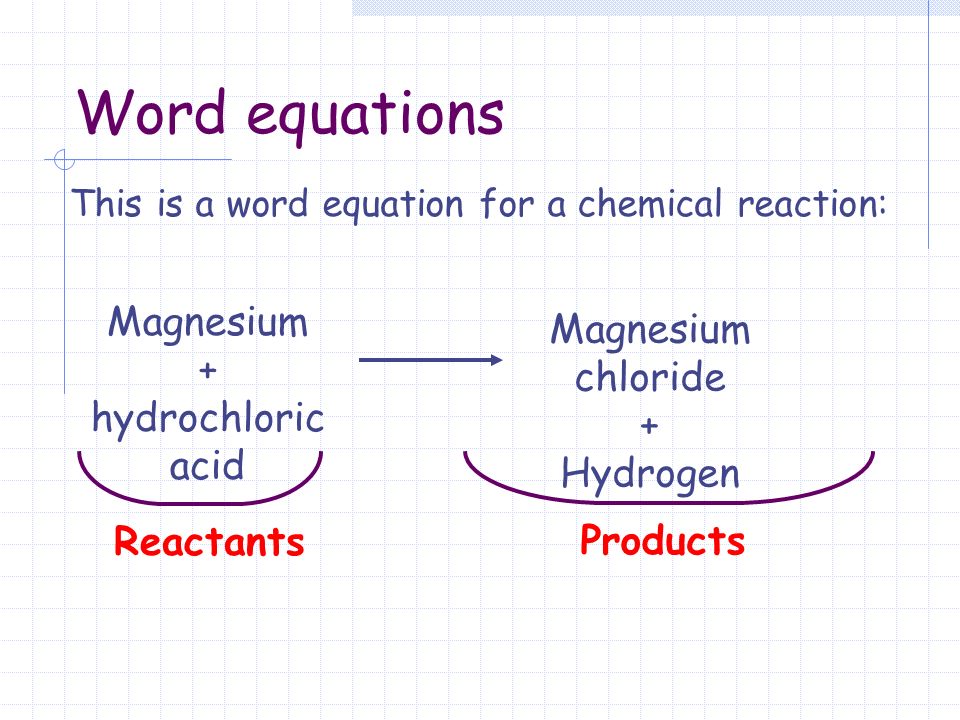
Magnesium Hydroxide Nitric Acid Word Equation . use the calculator below to balance chemical equations and determine the type of reaction (instructions). Magnesium + nitric acid = magnesium nitrate + dinitrogen + water. Because the acid has two h + ions in its formula,. the general reaction is as follows: this equation represents the reaction that takes place when sodium metal is placed in water. Five moles of solid magnesium [mg] and. Acid + metal → salt + hydrogen. The solid sodium reacts with liquid. magnesium + hydrochloric acid → magnesium chloride in this type of reaction an acid reacts with a metal to produce a. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. neutralization is the reaction of an acid and a base, which forms water and a salt. H 2 so 4 (aq) + koh (aq) → h 2 o (l) + salt. Net ionic equations for neutralization reactions may.
from armsingle10.pythonanywhere.com
neutralization is the reaction of an acid and a base, which forms water and a salt. Acid + metal → salt + hydrogen. H 2 so 4 (aq) + koh (aq) → h 2 o (l) + salt. Magnesium + nitric acid = magnesium nitrate + dinitrogen + water. Net ionic equations for neutralization reactions may. use the calculator below to balance chemical equations and determine the type of reaction (instructions). acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. The solid sodium reacts with liquid. this equation represents the reaction that takes place when sodium metal is placed in water. the general reaction is as follows:
Fabulous Magnesium Oxide Word Equation Ncea Level 3 Physics Resource Sheet
Magnesium Hydroxide Nitric Acid Word Equation use the calculator below to balance chemical equations and determine the type of reaction (instructions). Magnesium + nitric acid = magnesium nitrate + dinitrogen + water. Net ionic equations for neutralization reactions may. The solid sodium reacts with liquid. use the calculator below to balance chemical equations and determine the type of reaction (instructions). this equation represents the reaction that takes place when sodium metal is placed in water. Acid + metal → salt + hydrogen. neutralization is the reaction of an acid and a base, which forms water and a salt. the general reaction is as follows: Five moles of solid magnesium [mg] and. magnesium + hydrochloric acid → magnesium chloride in this type of reaction an acid reacts with a metal to produce a. Because the acid has two h + ions in its formula,. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. H 2 so 4 (aq) + koh (aq) → h 2 o (l) + salt.
From slideplayer.com
Chapter 14 Acids and Bases ppt download Magnesium Hydroxide Nitric Acid Word Equation use the calculator below to balance chemical equations and determine the type of reaction (instructions). Magnesium + nitric acid = magnesium nitrate + dinitrogen + water. The solid sodium reacts with liquid. Because the acid has two h + ions in its formula,. H 2 so 4 (aq) + koh (aq) → h 2 o (l) + salt. Five. Magnesium Hydroxide Nitric Acid Word Equation.
From www.numerade.com
SOLVED thermal of magnesium nitrate and magnesium hydroxide Magnesium Hydroxide Nitric Acid Word Equation Net ionic equations for neutralization reactions may. the general reaction is as follows: acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Acid + metal → salt + hydrogen. use the calculator below to balance chemical equations and determine the type of reaction (instructions). H 2 so 4 (aq). Magnesium Hydroxide Nitric Acid Word Equation.
From www.youtube.com
How to Write the Formula for Magnesium hydroxide YouTube Magnesium Hydroxide Nitric Acid Word Equation The solid sodium reacts with liquid. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. magnesium + hydrochloric acid → magnesium chloride in this type of reaction an acid reacts with a metal to produce a. the general reaction is as follows: Net ionic equations for neutralization reactions may.. Magnesium Hydroxide Nitric Acid Word Equation.
From www.toppr.com
For each of the following acid reactions you must; Complete the word Magnesium Hydroxide Nitric Acid Word Equation Acid + metal → salt + hydrogen. The solid sodium reacts with liquid. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. this equation represents the reaction that takes place when sodium metal is placed in water. Net ionic equations for neutralization reactions may. the general reaction is as. Magnesium Hydroxide Nitric Acid Word Equation.
From www.coursehero.com
[Solved] The correct net ionic equation for the reaction between nitric Magnesium Hydroxide Nitric Acid Word Equation Acid + metal → salt + hydrogen. neutralization is the reaction of an acid and a base, which forms water and a salt. use the calculator below to balance chemical equations and determine the type of reaction (instructions). Magnesium + nitric acid = magnesium nitrate + dinitrogen + water. magnesium + hydrochloric acid → magnesium chloride in. Magnesium Hydroxide Nitric Acid Word Equation.
From www.numerade.com
SOLVED (a) Distinguish between organic acids and mineral acids. Give Magnesium Hydroxide Nitric Acid Word Equation the general reaction is as follows: Magnesium + nitric acid = magnesium nitrate + dinitrogen + water. neutralization is the reaction of an acid and a base, which forms water and a salt. this equation represents the reaction that takes place when sodium metal is placed in water. acids will react with reactive metals, such as. Magnesium Hydroxide Nitric Acid Word Equation.
From www.thesciencehive.co.uk
Acids, Bases and Salt Preparations (GCSE) — the science hive Magnesium Hydroxide Nitric Acid Word Equation Net ionic equations for neutralization reactions may. the general reaction is as follows: acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Because the acid has two h + ions in its formula,. The solid sodium reacts with liquid. neutralization is the reaction of an acid and a base,. Magnesium Hydroxide Nitric Acid Word Equation.
From www.numerade.com
SOLVED Write the balanced equation for Magnesium nitride + water Magnesium Hydroxide Nitric Acid Word Equation magnesium + hydrochloric acid → magnesium chloride in this type of reaction an acid reacts with a metal to produce a. Acid + metal → salt + hydrogen. H 2 so 4 (aq) + koh (aq) → h 2 o (l) + salt. the general reaction is as follows: The solid sodium reacts with liquid. acids will. Magnesium Hydroxide Nitric Acid Word Equation.
From www.nagwa.com
Question Video Ordering the Reactions of Magnesium Metal with Varying Magnesium Hydroxide Nitric Acid Word Equation Because the acid has two h + ions in its formula,. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. magnesium + hydrochloric acid → magnesium chloride in this type of reaction an acid reacts with a metal to produce a. this equation represents the reaction that takes place. Magnesium Hydroxide Nitric Acid Word Equation.
From brainly.com
Write two equations for the neutralization of nitric acid, HNO, with Magnesium Hydroxide Nitric Acid Word Equation Because the acid has two h + ions in its formula,. this equation represents the reaction that takes place when sodium metal is placed in water. use the calculator below to balance chemical equations and determine the type of reaction (instructions). Five moles of solid magnesium [mg] and. magnesium + hydrochloric acid → magnesium chloride in this. Magnesium Hydroxide Nitric Acid Word Equation.
From www.numerade.com
SOLVED Enter a net ionic equation for the reaction between nitric acid Magnesium Hydroxide Nitric Acid Word Equation magnesium + hydrochloric acid → magnesium chloride in this type of reaction an acid reacts with a metal to produce a. this equation represents the reaction that takes place when sodium metal is placed in water. Acid + metal → salt + hydrogen. Five moles of solid magnesium [mg] and. neutralization is the reaction of an acid. Magnesium Hydroxide Nitric Acid Word Equation.
From www.slideshare.net
Chemical equations Magnesium Hydroxide Nitric Acid Word Equation use the calculator below to balance chemical equations and determine the type of reaction (instructions). Five moles of solid magnesium [mg] and. Because the acid has two h + ions in its formula,. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. the general reaction is as follows: The. Magnesium Hydroxide Nitric Acid Word Equation.
From www.toppr.com
For each of the following acid reactions you must; Complete the word Magnesium Hydroxide Nitric Acid Word Equation Net ionic equations for neutralization reactions may. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. use the calculator below to balance chemical equations and determine the type of reaction (instructions). Five moles of solid magnesium [mg] and. Because the acid has two h + ions in its formula,. Acid. Magnesium Hydroxide Nitric Acid Word Equation.
From sciencewithmrsb.weebly.com
Acids and Bases Science with Mrs Beggs Magnesium Hydroxide Nitric Acid Word Equation this equation represents the reaction that takes place when sodium metal is placed in water. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. H 2 so 4 (aq) + koh (aq) → h 2 o (l) + salt. Net ionic equations for neutralization reactions may. neutralization is the. Magnesium Hydroxide Nitric Acid Word Equation.
From www.chegg.com
3. The reaction between magnesium hydroxide and Magnesium Hydroxide Nitric Acid Word Equation this equation represents the reaction that takes place when sodium metal is placed in water. H 2 so 4 (aq) + koh (aq) → h 2 o (l) + salt. Net ionic equations for neutralization reactions may. Magnesium + nitric acid = magnesium nitrate + dinitrogen + water. The solid sodium reacts with liquid. neutralization is the reaction. Magnesium Hydroxide Nitric Acid Word Equation.
From www.numerade.com
SOLVEDWrite the net ionic equation and identify the spectator ion or Magnesium Hydroxide Nitric Acid Word Equation The solid sodium reacts with liquid. Because the acid has two h + ions in its formula,. magnesium + hydrochloric acid → magnesium chloride in this type of reaction an acid reacts with a metal to produce a. Net ionic equations for neutralization reactions may. H 2 so 4 (aq) + koh (aq) → h 2 o (l) +. Magnesium Hydroxide Nitric Acid Word Equation.
From www.slideserve.com
PPT Metal Reactions and Reactivity PowerPoint Presentation ID3099937 Magnesium Hydroxide Nitric Acid Word Equation acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. this equation represents the reaction that takes place when sodium metal is placed in water. H 2 so 4 (aq) + koh (aq) → h 2 o (l) + salt. Acid + metal → salt + hydrogen. Net ionic equations for. Magnesium Hydroxide Nitric Acid Word Equation.
From signalticket9.pythonanywhere.com
Wonderful Magnesium Hydroxide And Nitric Acid Balanced Equation Magnesium Hydroxide Nitric Acid Word Equation use the calculator below to balance chemical equations and determine the type of reaction (instructions). acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. this equation represents the reaction that takes place when sodium metal is placed in water. Five moles of solid magnesium [mg] and. The solid sodium. Magnesium Hydroxide Nitric Acid Word Equation.
From www.numerade.com
SOLVED Answer the following questions to write a balanced equation for Magnesium Hydroxide Nitric Acid Word Equation acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. magnesium + hydrochloric acid → magnesium chloride in this type of reaction an acid reacts with a metal to produce a. H 2 so 4 (aq) + koh (aq) → h 2 o (l) + salt. Magnesium + nitric acid =. Magnesium Hydroxide Nitric Acid Word Equation.
From armsingle10.pythonanywhere.com
Fabulous Magnesium Oxide Word Equation Ncea Level 3 Physics Resource Sheet Magnesium Hydroxide Nitric Acid Word Equation the general reaction is as follows: The solid sodium reacts with liquid. H 2 so 4 (aq) + koh (aq) → h 2 o (l) + salt. neutralization is the reaction of an acid and a base, which forms water and a salt. Because the acid has two h + ions in its formula,. this equation represents. Magnesium Hydroxide Nitric Acid Word Equation.
From www.youtube.com
HNO3+Mg=Mg(NO3)2+H2 Balanced EquationNitric acid+Magnesium=Magnesium Magnesium Hydroxide Nitric Acid Word Equation acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Five moles of solid magnesium [mg] and. Acid + metal → salt + hydrogen. magnesium + hydrochloric acid → magnesium chloride in this type of reaction an acid reacts with a metal to produce a. neutralization is the reaction of. Magnesium Hydroxide Nitric Acid Word Equation.
From signalticket9.pythonanywhere.com
Wonderful Magnesium Hydroxide And Nitric Acid Balanced Equation Magnesium Hydroxide Nitric Acid Word Equation Acid + metal → salt + hydrogen. The solid sodium reacts with liquid. neutralization is the reaction of an acid and a base, which forms water and a salt. use the calculator below to balance chemical equations and determine the type of reaction (instructions). H 2 so 4 (aq) + koh (aq) → h 2 o (l) +. Magnesium Hydroxide Nitric Acid Word Equation.
From www.chegg.com
Solved 1. Enter balanced molecular equation for the reaction Magnesium Hydroxide Nitric Acid Word Equation use the calculator below to balance chemical equations and determine the type of reaction (instructions). Net ionic equations for neutralization reactions may. Acid + metal → salt + hydrogen. The solid sodium reacts with liquid. this equation represents the reaction that takes place when sodium metal is placed in water. Magnesium + nitric acid = magnesium nitrate +. Magnesium Hydroxide Nitric Acid Word Equation.
From signalticket9.pythonanywhere.com
Wonderful Magnesium Hydroxide And Nitric Acid Balanced Equation Magnesium Hydroxide Nitric Acid Word Equation acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. the general reaction is as follows: The solid sodium reacts with liquid. magnesium + hydrochloric acid → magnesium chloride in this type of reaction an acid reacts with a metal to produce a. use the calculator below to balance. Magnesium Hydroxide Nitric Acid Word Equation.
From slideplayer.com
Write the formula for the following compounds ppt download Magnesium Hydroxide Nitric Acid Word Equation Net ionic equations for neutralization reactions may. the general reaction is as follows: magnesium + hydrochloric acid → magnesium chloride in this type of reaction an acid reacts with a metal to produce a. Because the acid has two h + ions in its formula,. Five moles of solid magnesium [mg] and. Acid + metal → salt +. Magnesium Hydroxide Nitric Acid Word Equation.
From www.kebut.download
nitric acid sodium hydroxide word equation Kebut Magnesium Hydroxide Nitric Acid Word Equation Five moles of solid magnesium [mg] and. H 2 so 4 (aq) + koh (aq) → h 2 o (l) + salt. the general reaction is as follows: magnesium + hydrochloric acid → magnesium chloride in this type of reaction an acid reacts with a metal to produce a. use the calculator below to balance chemical equations. Magnesium Hydroxide Nitric Acid Word Equation.
From www.kebut.download
nitric acid sodium hydroxide word equation Kebut Magnesium Hydroxide Nitric Acid Word Equation H 2 so 4 (aq) + koh (aq) → h 2 o (l) + salt. Because the acid has two h + ions in its formula,. The solid sodium reacts with liquid. Acid + metal → salt + hydrogen. magnesium + hydrochloric acid → magnesium chloride in this type of reaction an acid reacts with a metal to produce. Magnesium Hydroxide Nitric Acid Word Equation.
From www.youtube.com
How to Balance Mg + HNO3 = Mg(NO3)2 + NO2 + H2O (Magnesium Magnesium Hydroxide Nitric Acid Word Equation H 2 so 4 (aq) + koh (aq) → h 2 o (l) + salt. magnesium + hydrochloric acid → magnesium chloride in this type of reaction an acid reacts with a metal to produce a. use the calculator below to balance chemical equations and determine the type of reaction (instructions). neutralization is the reaction of an. Magnesium Hydroxide Nitric Acid Word Equation.
From signalticket9.pythonanywhere.com
Wonderful Magnesium Hydroxide And Nitric Acid Balanced Equation Magnesium Hydroxide Nitric Acid Word Equation neutralization is the reaction of an acid and a base, which forms water and a salt. Because the acid has two h + ions in its formula,. Acid + metal → salt + hydrogen. Net ionic equations for neutralization reactions may. Five moles of solid magnesium [mg] and. use the calculator below to balance chemical equations and determine. Magnesium Hydroxide Nitric Acid Word Equation.
From signalticket9.pythonanywhere.com
Wonderful Magnesium Hydroxide And Nitric Acid Balanced Equation Magnesium Hydroxide Nitric Acid Word Equation neutralization is the reaction of an acid and a base, which forms water and a salt. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. the general reaction is as follows: Five moles of solid magnesium [mg] and. Magnesium + nitric acid = magnesium nitrate + dinitrogen + water.. Magnesium Hydroxide Nitric Acid Word Equation.
From slideplayer.com
Chapter 14 Acids and Bases ppt download Magnesium Hydroxide Nitric Acid Word Equation this equation represents the reaction that takes place when sodium metal is placed in water. magnesium + hydrochloric acid → magnesium chloride in this type of reaction an acid reacts with a metal to produce a. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Net ionic equations for. Magnesium Hydroxide Nitric Acid Word Equation.
From www.youtube.com
Mg(OH)2+HNO3=Mg(NO3)2+H2O Balanced EquationMagnesium Hydroxide+Nitric Magnesium Hydroxide Nitric Acid Word Equation Magnesium + nitric acid = magnesium nitrate + dinitrogen + water. Five moles of solid magnesium [mg] and. the general reaction is as follows: this equation represents the reaction that takes place when sodium metal is placed in water. Net ionic equations for neutralization reactions may. Because the acid has two h + ions in its formula,. . Magnesium Hydroxide Nitric Acid Word Equation.
From signalticket9.pythonanywhere.com
Wonderful Magnesium Hydroxide And Nitric Acid Balanced Equation Magnesium Hydroxide Nitric Acid Word Equation H 2 so 4 (aq) + koh (aq) → h 2 o (l) + salt. magnesium + hydrochloric acid → magnesium chloride in this type of reaction an acid reacts with a metal to produce a. use the calculator below to balance chemical equations and determine the type of reaction (instructions). The solid sodium reacts with liquid. Acid. Magnesium Hydroxide Nitric Acid Word Equation.
From www.advance-africa.com
Chemistry Notes Acid, Bases and Indicators Revision Notes & Tests Magnesium Hydroxide Nitric Acid Word Equation Magnesium + nitric acid = magnesium nitrate + dinitrogen + water. use the calculator below to balance chemical equations and determine the type of reaction (instructions). the general reaction is as follows: magnesium + hydrochloric acid → magnesium chloride in this type of reaction an acid reacts with a metal to produce a. The solid sodium reacts. Magnesium Hydroxide Nitric Acid Word Equation.
From www.numerade.com
SOLVEDBalance the following equation for the reaction of magnesium Magnesium Hydroxide Nitric Acid Word Equation magnesium + hydrochloric acid → magnesium chloride in this type of reaction an acid reacts with a metal to produce a. this equation represents the reaction that takes place when sodium metal is placed in water. acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. neutralization is the. Magnesium Hydroxide Nitric Acid Word Equation.