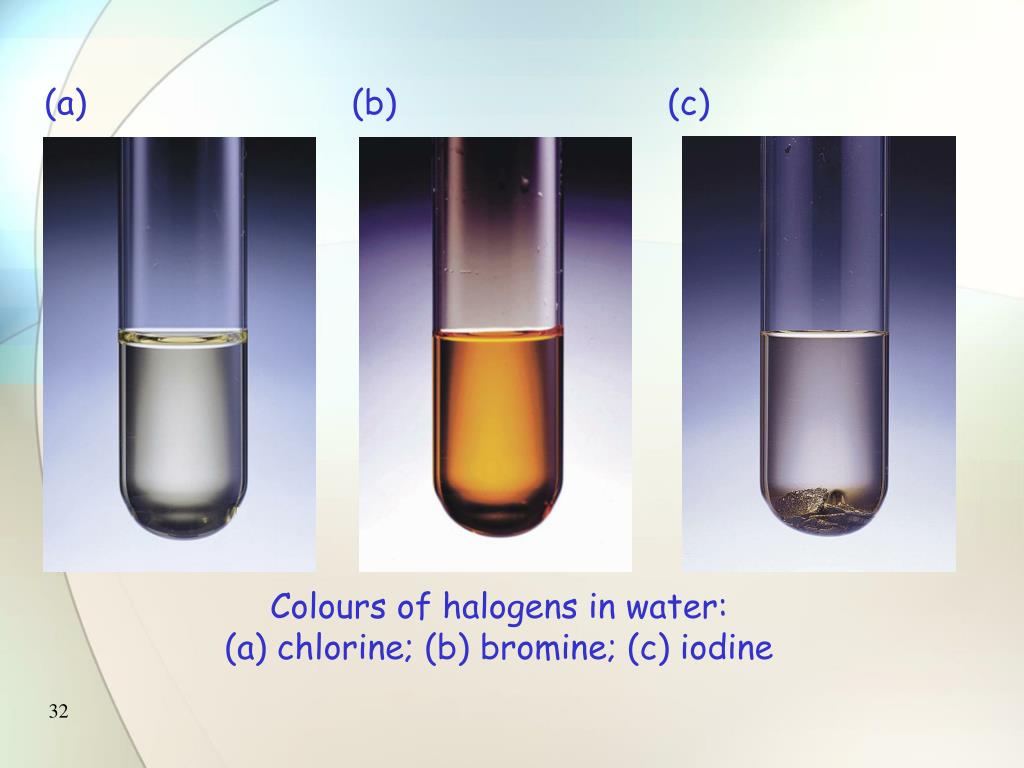
Chlorine Colour Solution . Chlorine with bromides & iodides. The relative oxidizing strengths of chlorine can be illustrated nicely in the laboratory. This brown colour is the. If, for example, a solution of cl 2 in h 2 o is combined with a. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement. Chlorine is also more reactive than the bromine in potassium bromide solution:. For chlorine and bromine the colour does not change. However, for iodine there is a colour change, from brown. Because chlorine is more reactive than bromine, it displaces bromine from sodium bromide. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: Chlorine with bromides & iodides. Swedish chemist carl wilhelm scheele produced chlorine in 1774 by the reaction of manganese dioxide (mno 2 ) with a solution of hydrochloric. The colourless solution changes to brown. You might need a white background to see the colour of the chlorine solution.
from www.slideserve.com
The relative oxidizing strengths of chlorine can be illustrated nicely in the laboratory. However, for iodine there is a colour change, from brown. Because chlorine is more reactive than bromine, it displaces bromine from sodium bromide. Chlorine with bromides & iodides. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: You might need a white background to see the colour of the chlorine solution. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement. Swedish chemist carl wilhelm scheele produced chlorine in 1774 by the reaction of manganese dioxide (mno 2 ) with a solution of hydrochloric. Chlorine is also more reactive than the bromine in potassium bromide solution:. For chlorine and bromine the colour does not change.
PPT Characteristic Properties of the Halogens PowerPoint Presentation
Chlorine Colour Solution However, for iodine there is a colour change, from brown. This brown colour is the. You might need a white background to see the colour of the chlorine solution. Swedish chemist carl wilhelm scheele produced chlorine in 1774 by the reaction of manganese dioxide (mno 2 ) with a solution of hydrochloric. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement. Chlorine with bromides & iodides. The colourless solution changes to brown. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: Chlorine is also more reactive than the bromine in potassium bromide solution:. Chlorine with bromides & iodides. However, for iodine there is a colour change, from brown. Because chlorine is more reactive than bromine, it displaces bromine from sodium bromide. If, for example, a solution of cl 2 in h 2 o is combined with a. For chlorine and bromine the colour does not change. The relative oxidizing strengths of chlorine can be illustrated nicely in the laboratory.
From ar.inspiredpencil.com
Chlorine Gas Colour Chlorine Colour Solution Chlorine with bromides & iodides. The relative oxidizing strengths of chlorine can be illustrated nicely in the laboratory. Chlorine is also more reactive than the bromine in potassium bromide solution:. Chlorine with bromides & iodides. This brown colour is the. For chlorine and bromine the colour does not change. You might need a white background to see the colour of. Chlorine Colour Solution.
From www.slideshare.net
Oxidation & reduction Chlorine Colour Solution However, for iodine there is a colour change, from brown. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: You might need a white background to see the colour of the chlorine solution. Because chlorine is more reactive than bromine, it displaces bromine from sodium bromide. If you add chlorine solution to. Chlorine Colour Solution.
From www.sciencephoto.com
Copper (II) chloride solution Stock Image C029/5916 Science Photo Chlorine Colour Solution You might need a white background to see the colour of the chlorine solution. Chlorine with bromides & iodides. The relative oxidizing strengths of chlorine can be illustrated nicely in the laboratory. This brown colour is the. For chlorine and bromine the colour does not change. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a. Chlorine Colour Solution.
From www.bunnings.com.au
HyClor 5L Liquid Chlorine Bunnings Warehouse Chlorine Colour Solution Chlorine with bromides & iodides. If, for example, a solution of cl 2 in h 2 o is combined with a. This brown colour is the. However, for iodine there is a colour change, from brown. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: The relative oxidizing strengths of chlorine can. Chlorine Colour Solution.
From www.sciencephoto.com
Cobalt Chloride Stock Image C002/8003 Science Photo Library Chlorine Colour Solution Swedish chemist carl wilhelm scheele produced chlorine in 1774 by the reaction of manganese dioxide (mno 2 ) with a solution of hydrochloric. You might need a white background to see the colour of the chlorine solution. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: The relative oxidizing strengths of chlorine. Chlorine Colour Solution.
From www.slideserve.com
PPT Characteristic Properties of the Halogens PowerPoint Presentation Chlorine Colour Solution Swedish chemist carl wilhelm scheele produced chlorine in 1774 by the reaction of manganese dioxide (mno 2 ) with a solution of hydrochloric. However, for iodine there is a colour change, from brown. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement. You might need a white background to see the colour of the. Chlorine Colour Solution.
From www.chemedx.org
Demonstrating the Colors of Transition Metal Complex Ions Chemical Chlorine Colour Solution Chlorine with bromides & iodides. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement. If, for example, a solution of cl 2 in h 2 o is combined with a. The relative oxidizing strengths of chlorine can be illustrated nicely in the laboratory. For chlorine and bromine the colour does not change. This brown. Chlorine Colour Solution.
From fineartamerica.com
Iron Chlorides Photograph by Martyn F. Chillmaid/science Photo Library Chlorine Colour Solution This brown colour is the. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement. For chlorine and bromine the colour does not change. Chlorine with bromides & iodides. You might need a white background to see the colour of the chlorine solution. Swedish chemist carl wilhelm scheele produced chlorine in 1774 by the reaction. Chlorine Colour Solution.
From jamiya-has-robertson.blogspot.com
Colour of Silver Chloride JamiyahasRobertson Chlorine Colour Solution If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement. Swedish chemist carl wilhelm scheele produced chlorine in 1774 by the reaction of manganese dioxide (mno 2 ) with a solution of hydrochloric. Chlorine with bromides & iodides. However, for iodine there is a colour change, from brown. You might need a white background to. Chlorine Colour Solution.
From www.youtube.com
How to make a Stannous Chloride Solution (Tin (II) Chloride) YouTube Chlorine Colour Solution You might need a white background to see the colour of the chlorine solution. Chlorine with bromides & iodides. Because chlorine is more reactive than bromine, it displaces bromine from sodium bromide. However, for iodine there is a colour change, from brown. This brown colour is the. Chlorine is also more reactive than the bromine in potassium bromide solution:. The. Chlorine Colour Solution.
From waterislifeonline.blogspot.com
How To Use Chlorine Test Solution. Water Is Life Blog Chlorine Colour Solution Swedish chemist carl wilhelm scheele produced chlorine in 1774 by the reaction of manganese dioxide (mno 2 ) with a solution of hydrochloric. This brown colour is the. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement. Chlorine with bromides & iodides. You might need a white background to see the colour of the. Chlorine Colour Solution.
From commons.wikimedia.org
FileChlorine dioxide solution.jpg Chlorine Colour Solution The relative oxidizing strengths of chlorine can be illustrated nicely in the laboratory. However, for iodine there is a colour change, from brown. This brown colour is the. Because chlorine is more reactive than bromine, it displaces bromine from sodium bromide. If, for example, a solution of cl 2 in h 2 o is combined with a. Chlorine with bromides. Chlorine Colour Solution.
From seguridadbiologica.blogspot.com
ShelfLife of Chlorine Solutions in Ebola Virus Disease Chlorine Colour Solution Swedish chemist carl wilhelm scheele produced chlorine in 1774 by the reaction of manganese dioxide (mno 2 ) with a solution of hydrochloric. However, for iodine there is a colour change, from brown. The relative oxidizing strengths of chlorine can be illustrated nicely in the laboratory. For chlorine and bromine the colour does not change. You might need a white. Chlorine Colour Solution.
From fphoto.photoshelter.com
science chemistry halide displacement reaction chlorine postassium Chlorine Colour Solution Chlorine is also more reactive than the bromine in potassium bromide solution:. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement. Chlorine with bromides & iodides. Because chlorine is more reactive than bromine, it displaces bromine from sodium bromide. Chlorine with bromides & iodides. However, for iodine there is a colour change, from brown.. Chlorine Colour Solution.
From www.chegg.com
Solved A. Answer Hypotheses questions 1A1D on page 1 of Chlorine Colour Solution The relative oxidizing strengths of chlorine can be illustrated nicely in the laboratory. You might need a white background to see the colour of the chlorine solution. However, for iodine there is a colour change, from brown. Chlorine with bromides & iodides. Chlorine with bromides & iodides. Swedish chemist carl wilhelm scheele produced chlorine in 1774 by the reaction of. Chlorine Colour Solution.
From cartoondealer.com
Chlorine Stock Photography 10272668 Chlorine Colour Solution This brown colour is the. You might need a white background to see the colour of the chlorine solution. Because chlorine is more reactive than bromine, it displaces bromine from sodium bromide. For chlorine and bromine the colour does not change. However, for iodine there is a colour change, from brown. Chlorine with bromides & iodides. Chlorine is also more. Chlorine Colour Solution.
From www.slideserve.com
PPT Transition Metal Chemistry and Coordination Compounds PowerPoint Chlorine Colour Solution Chlorine with bromides & iodides. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement. If, for example, a solution of cl 2 in h 2 o is combined with a. You might need a white background to see the colour of the chlorine solution. This brown colour is the. Chlorine with bromides & iodides.. Chlorine Colour Solution.
From brainly.in
cupric chloride witch colour Brainly.in Chlorine Colour Solution If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement. Chlorine with bromides & iodides. The colourless solution changes to brown. This brown colour is the. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: However, for iodine there is a colour change, from brown. Chlorine with. Chlorine Colour Solution.
From www.chlorinetechservices.co.za
protective clothing and equipment Chlorine Technical Services South Chlorine Colour Solution This brown colour is the. However, for iodine there is a colour change, from brown. You might need a white background to see the colour of the chlorine solution. Swedish chemist carl wilhelm scheele produced chlorine in 1774 by the reaction of manganese dioxide (mno 2 ) with a solution of hydrochloric. Because chlorine is more reactive than bromine, it. Chlorine Colour Solution.
From www.indiamart.com
Liquid Chlorine Solution, For Industrial, Rs 30 /litre, Shree Arbuda Chlorine Colour Solution However, for iodine there is a colour change, from brown. Because chlorine is more reactive than bromine, it displaces bromine from sodium bromide. The colourless solution changes to brown. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: This brown colour is the. Swedish chemist carl wilhelm scheele produced chlorine in 1774. Chlorine Colour Solution.
From www.fishersci.com
Hach Company Chlorine Standard Solution, 5075 mg/L as Cl2, pk/16 10 Chlorine Colour Solution This brown colour is the. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement. Chlorine with bromides & iodides. Swedish chemist carl wilhelm scheele produced chlorine in 1774 by the reaction of manganese dioxide (mno 2 ) with a solution of hydrochloric. For chlorine and bromine the colour does not change. Chlorine is also. Chlorine Colour Solution.
From www.labkafe.com
Color of Common Salts Used in School Laboratories Labkafe Chlorine Colour Solution If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement. For chlorine and bromine the colour does not change. You might need a white background to see the colour of the chlorine solution. Swedish chemist carl wilhelm scheele. Chlorine Colour Solution.
From www.flinnsci.com
Cobalt Chloride Solution, 0.1 M, 500 mL Flinn Scientific Chlorine Colour Solution Chlorine is also more reactive than the bromine in potassium bromide solution:. However, for iodine there is a colour change, from brown. Chlorine with bromides & iodides. The relative oxidizing strengths of chlorine can be illustrated nicely in the laboratory. Because chlorine is more reactive than bromine, it displaces bromine from sodium bromide. This brown colour is the. Swedish chemist. Chlorine Colour Solution.
From www.youtube.com
Ferric Chloride Color, Solubility in Water FeCl3 Ion(III) chloride Chlorine Colour Solution For chlorine and bromine the colour does not change. Chlorine with bromides & iodides. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement. Swedish chemist carl wilhelm scheele produced chlorine in 1774 by the reaction of manganese dioxide (mno 2 ) with a solution of hydrochloric. Because chlorine is more reactive than bromine, it. Chlorine Colour Solution.
From www.slideshare.net
Redox Chlorine Colour Solution The relative oxidizing strengths of chlorine can be illustrated nicely in the laboratory. The colourless solution changes to brown. This brown colour is the. However, for iodine there is a colour change, from brown. Because chlorine is more reactive than bromine, it displaces bromine from sodium bromide. If you add chlorine solution to colourless potassium bromide or potassium iodide solution. Chlorine Colour Solution.
From classfullbrauer.z19.web.core.windows.net
Oto Chlorine Test Color Chart Chlorine Colour Solution Because chlorine is more reactive than bromine, it displaces bromine from sodium bromide. Swedish chemist carl wilhelm scheele produced chlorine in 1774 by the reaction of manganese dioxide (mno 2 ) with a solution of hydrochloric. However, for iodine there is a colour change, from brown. The colourless solution changes to brown. You might need a white background to see. Chlorine Colour Solution.
From www.youtube.com
How to make Stannous Chloride Solution SNCL2 (Tin (II) Chloride) .Easy Chlorine Colour Solution Swedish chemist carl wilhelm scheele produced chlorine in 1774 by the reaction of manganese dioxide (mno 2 ) with a solution of hydrochloric. If, for example, a solution of cl 2 in h 2 o is combined with a. You might need a white background to see the colour of the chlorine solution. Chlorine is also more reactive than the. Chlorine Colour Solution.
From edu.rsc.org
Halogens in aqueous solution and their displacement reactions Chlorine Colour Solution Chlorine is also more reactive than the bromine in potassium bromide solution:. Chlorine with bromides & iodides. This brown colour is the. Swedish chemist carl wilhelm scheele produced chlorine in 1774 by the reaction of manganese dioxide (mno 2 ) with a solution of hydrochloric. Chlorine with bromides & iodides. You might need a white background to see the colour. Chlorine Colour Solution.
From www.chemistrylearner.com
Chlorine Facts, Symbol, Discovery, Properties, Uses Chlorine Colour Solution The colourless solution changes to brown. However, for iodine there is a colour change, from brown. If, for example, a solution of cl 2 in h 2 o is combined with a. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement. Swedish chemist carl wilhelm scheele produced chlorine in 1774 by the reaction of. Chlorine Colour Solution.
From www.sciencephoto.com
Chlorine displacing iodine Stock Image A500/0386 Science Photo Chlorine Colour Solution If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: If, for example, a solution of cl 2 in h 2 o is combined with a. The colourless solution changes to brown. The relative oxidizing strengths of chlorine can be illustrated nicely in the laboratory. Swedish chemist carl wilhelm scheele produced chlorine in. Chlorine Colour Solution.
From www.indiamart.com
Chlorine Solution at Rs 5/kg 7782505, तरल क्लोरीन Davis Chemical Chlorine Colour Solution If, for example, a solution of cl 2 in h 2 o is combined with a. Chlorine with bromides & iodides. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement reaction occurs: Because chlorine is more reactive than bromine, it displaces bromine from sodium bromide. Swedish chemist carl wilhelm scheele produced chlorine in 1774. Chlorine Colour Solution.
From www.sycamorelifesciences.com
Chloride Color Reagent Solution, 4 L, Ricca 19401 New Laboratory Setup Chlorine Colour Solution Chlorine with bromides & iodides. Swedish chemist carl wilhelm scheele produced chlorine in 1774 by the reaction of manganese dioxide (mno 2 ) with a solution of hydrochloric. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement. You might need a white background to see the colour of the chlorine solution. The colourless solution. Chlorine Colour Solution.
From medica.org.pk
Chlorine solution 1000ml Medica Group of Industries Pvt. Limited Chlorine Colour Solution Chlorine with bromides & iodides. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement. If, for example, a solution of cl 2 in h 2 o is combined with a. You might need a white background to see the colour of the chlorine solution. Swedish chemist carl wilhelm scheele produced chlorine in 1774 by. Chlorine Colour Solution.
From www.youtube.com
Residual chlorine Experiment YouTube Chlorine Colour Solution Chlorine with bromides & iodides. Chlorine with bromides & iodides. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement. The relative oxidizing strengths of chlorine can be illustrated nicely in the laboratory. For chlorine and bromine the colour does not change. If, for example, a solution of cl 2 in h 2 o is. Chlorine Colour Solution.
From www.youtube.com
Chlorine Water + Sodium Bromide YouTube Chlorine Colour Solution This brown colour is the. However, for iodine there is a colour change, from brown. The relative oxidizing strengths of chlorine can be illustrated nicely in the laboratory. For chlorine and bromine the colour does not change. Chlorine is also more reactive than the bromine in potassium bromide solution:. Chlorine with bromides & iodides. Chlorine with bromides & iodides. If. Chlorine Colour Solution.