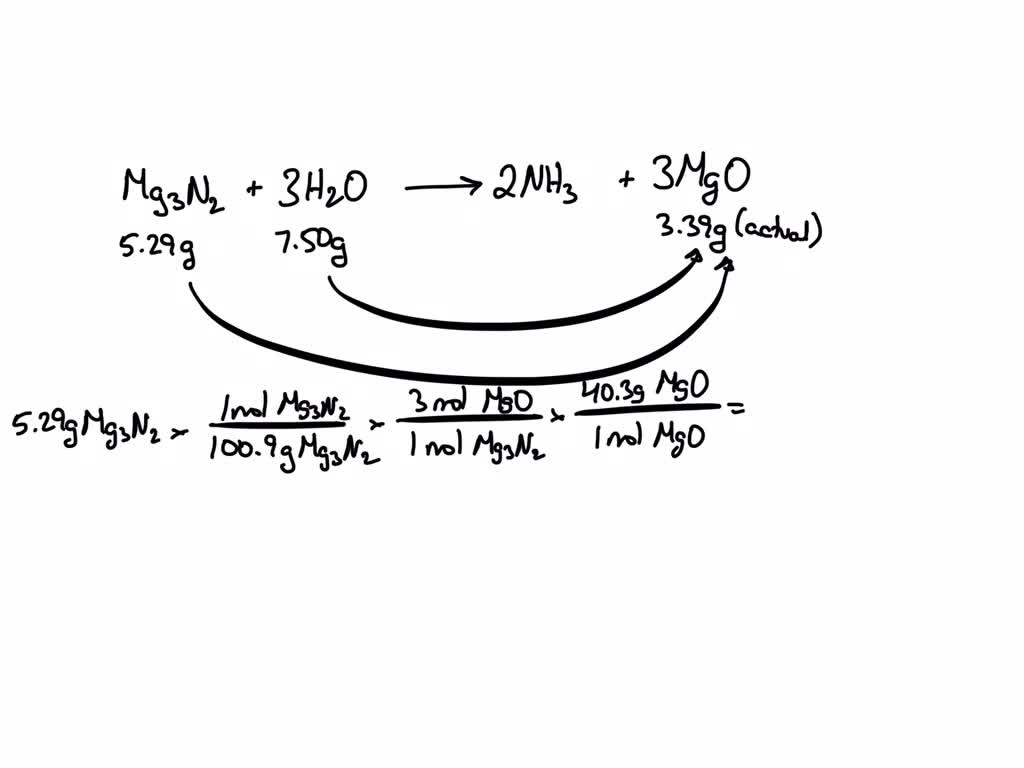
Magnesium Oxide Theoretical Yield . The maximum amount of product(s) that can be obtained in a reaction from a given amount of reactant(s) is the theoretical yield of the. Magnesium is reacted with oxygen from the air in a crucible, and the masses before and after the oxidation are measured. Percent yield = actual yield/theoretical yield x 100% percent yield = 15 g/19 g x 100% percent yield = 79% Then we will use the. 2mg (s) + o2(g) → 2mgo. What is the percent yield of magnesium oxide? This theoretical yield calculator will answer all the burning questions you have regarding how to calculate the theoretical yield,. Also determine the amount of excess reactant. The theoretical yield is 19 grams. Here, you know the actual yield (15 grams) and the theoretical yield (19 grams), so just plug the values into the formula: Mgco 3 → mgo + co 2. Magnesium oxide can be made by heating magnesium metal in the presence of oxygen. For example, in the reaction of magnesium metal and oxygen, calculate the mass of magnesium oxide that can be produced if 2.40 g \(mg\) reacts with 10.0 g \(o_2\). We will use stoichiometry to determine the theoretical yield of magnesium oxide. \(\ce{mgo}\) is the only product in the reaction.
from www.numerade.com
Magnesium oxide can be made by heating magnesium metal in the presence of oxygen. Then we will use the. \(\ce{mgo}\) is the only product in the reaction. We will use stoichiometry to determine the theoretical yield of magnesium oxide. Magnesium is reacted with oxygen from the air in a crucible, and the masses before and after the oxidation are measured. Percent yield = actual yield/theoretical yield x 100% percent yield = 15 g/19 g x 100% percent yield = 79% This theoretical yield calculator will answer all the burning questions you have regarding how to calculate the theoretical yield,. Here, you know the actual yield (15 grams) and the theoretical yield (19 grams), so just plug the values into the formula: Also determine the amount of excess reactant. Mgco 3 → mgo + co 2.
SOLVED 'Experiment 8 QUANTITIES IN CHEMICAL REACTIONS PreLab
Magnesium Oxide Theoretical Yield The theoretical yield is 19 grams. Magnesium oxide can be made by heating magnesium metal in the presence of oxygen. The theoretical yield is 19 grams. \(\ce{mgo}\) is the only product in the reaction. Magnesium is reacted with oxygen from the air in a crucible, and the masses before and after the oxidation are measured. The maximum amount of product(s) that can be obtained in a reaction from a given amount of reactant(s) is the theoretical yield of the. This theoretical yield calculator will answer all the burning questions you have regarding how to calculate the theoretical yield,. Mgco 3 → mgo + co 2. Here, you know the actual yield (15 grams) and the theoretical yield (19 grams), so just plug the values into the formula: Then we will use the. Also determine the amount of excess reactant. What is the percent yield of magnesium oxide? 2mg (s) + o2(g) → 2mgo. For example, in the reaction of magnesium metal and oxygen, calculate the mass of magnesium oxide that can be produced if 2.40 g \(mg\) reacts with 10.0 g \(o_2\). Percent yield = actual yield/theoretical yield x 100% percent yield = 15 g/19 g x 100% percent yield = 79% We will use stoichiometry to determine the theoretical yield of magnesium oxide.
From www.numerade.com
When 10.1g of magnesium (Mg) reacts with 10.5g of oxygen gas (O2), the Magnesium Oxide Theoretical Yield This theoretical yield calculator will answer all the burning questions you have regarding how to calculate the theoretical yield,. What is the percent yield of magnesium oxide? Here, you know the actual yield (15 grams) and the theoretical yield (19 grams), so just plug the values into the formula: The theoretical yield is 19 grams. 2mg (s) + o2(g) →. Magnesium Oxide Theoretical Yield.
From slideplayer.com
Theoretical Yield & Percent Yield ppt download Magnesium Oxide Theoretical Yield Magnesium is reacted with oxygen from the air in a crucible, and the masses before and after the oxidation are measured. 2mg (s) + o2(g) → 2mgo. \(\ce{mgo}\) is the only product in the reaction. We will use stoichiometry to determine the theoretical yield of magnesium oxide. Then we will use the. Also determine the amount of excess reactant. Magnesium. Magnesium Oxide Theoretical Yield.
From www.numerade.com
SOLVEDMagnesium oxide can be made by heating magnesium metal in the Magnesium Oxide Theoretical Yield Magnesium oxide can be made by heating magnesium metal in the presence of oxygen. Then we will use the. Percent yield = actual yield/theoretical yield x 100% percent yield = 15 g/19 g x 100% percent yield = 79% Here, you know the actual yield (15 grams) and the theoretical yield (19 grams), so just plug the values into the. Magnesium Oxide Theoretical Yield.
From www.numerade.com
SOLVEDData Mass of crucible, lid and turnings Mass of empty crucible Magnesium Oxide Theoretical Yield Here, you know the actual yield (15 grams) and the theoretical yield (19 grams), so just plug the values into the formula: Mgco 3 → mgo + co 2. Percent yield = actual yield/theoretical yield x 100% percent yield = 15 g/19 g x 100% percent yield = 79% Magnesium oxide can be made by heating magnesium metal in the. Magnesium Oxide Theoretical Yield.
From www.solutionspile.com
[Solved] A ( 0.236 mathrm{g} ) piece of solid magnesiu Magnesium Oxide Theoretical Yield Also determine the amount of excess reactant. 2mg (s) + o2(g) → 2mgo. Magnesium is reacted with oxygen from the air in a crucible, and the masses before and after the oxidation are measured. This theoretical yield calculator will answer all the burning questions you have regarding how to calculate the theoretical yield,. Here, you know the actual yield (15. Magnesium Oxide Theoretical Yield.
From slideplayer.com
Chemistry Final Exam review ppt download Magnesium Oxide Theoretical Yield 2mg (s) + o2(g) → 2mgo. Magnesium is reacted with oxygen from the air in a crucible, and the masses before and after the oxidation are measured. Here, you know the actual yield (15 grams) and the theoretical yield (19 grams), so just plug the values into the formula: Mgco 3 → mgo + co 2. For example, in the. Magnesium Oxide Theoretical Yield.
From www.numerade.com
SOLVED 2.59g of mg reacts with 7.59g of oxygen to for magnesium exude Magnesium Oxide Theoretical Yield Here, you know the actual yield (15 grams) and the theoretical yield (19 grams), so just plug the values into the formula: Magnesium oxide can be made by heating magnesium metal in the presence of oxygen. Mgco 3 → mgo + co 2. We will use stoichiometry to determine the theoretical yield of magnesium oxide. \(\ce{mgo}\) is the only product. Magnesium Oxide Theoretical Yield.
From www.showme.com
Calculating Theoretical Yield MgO ShowMe Magnesium Oxide Theoretical Yield For example, in the reaction of magnesium metal and oxygen, calculate the mass of magnesium oxide that can be produced if 2.40 g \(mg\) reacts with 10.0 g \(o_2\). Then we will use the. Here, you know the actual yield (15 grams) and the theoretical yield (19 grams), so just plug the values into the formula: Also determine the amount. Magnesium Oxide Theoretical Yield.
From www.youtube.com
CHEM 101 Dimensional Analysis Limiting Reagent, Theoretical Yield Magnesium Oxide Theoretical Yield Magnesium oxide can be made by heating magnesium metal in the presence of oxygen. Percent yield = actual yield/theoretical yield x 100% percent yield = 15 g/19 g x 100% percent yield = 79% For example, in the reaction of magnesium metal and oxygen, calculate the mass of magnesium oxide that can be produced if 2.40 g \(mg\) reacts with. Magnesium Oxide Theoretical Yield.
From www.numerade.com
SOLVED Consider the following balanced equation 3Mg(OH)2(s) + 2H3PO4 Magnesium Oxide Theoretical Yield We will use stoichiometry to determine the theoretical yield of magnesium oxide. What is the percent yield of magnesium oxide? 2mg (s) + o2(g) → 2mgo. This theoretical yield calculator will answer all the burning questions you have regarding how to calculate the theoretical yield,. Magnesium is reacted with oxygen from the air in a crucible, and the masses before. Magnesium Oxide Theoretical Yield.
From www.numerade.com
Magnesium burns in air with a dazzling brilliance to produce magnesium Magnesium Oxide Theoretical Yield We will use stoichiometry to determine the theoretical yield of magnesium oxide. The theoretical yield is 19 grams. Percent yield = actual yield/theoretical yield x 100% percent yield = 15 g/19 g x 100% percent yield = 79% What is the percent yield of magnesium oxide? Here, you know the actual yield (15 grams) and the theoretical yield (19 grams),. Magnesium Oxide Theoretical Yield.
From www.numerade.com
SOLVED Magnesium oxide can be made by heating magnesium metal in the Magnesium Oxide Theoretical Yield This theoretical yield calculator will answer all the burning questions you have regarding how to calculate the theoretical yield,. For example, in the reaction of magnesium metal and oxygen, calculate the mass of magnesium oxide that can be produced if 2.40 g \(mg\) reacts with 10.0 g \(o_2\). What is the percent yield of magnesium oxide? Magnesium is reacted with. Magnesium Oxide Theoretical Yield.
From www.numerade.com
Magnesium oxide can be made by heating magnesium metal in the presence Magnesium Oxide Theoretical Yield We will use stoichiometry to determine the theoretical yield of magnesium oxide. This theoretical yield calculator will answer all the burning questions you have regarding how to calculate the theoretical yield,. Here, you know the actual yield (15 grams) and the theoretical yield (19 grams), so just plug the values into the formula: Magnesium oxide can be made by heating. Magnesium Oxide Theoretical Yield.
From www.numerade.com
SOLVED Review Constants Periodic Table Part A Magnesium oxide can be Magnesium Oxide Theoretical Yield The theoretical yield is 19 grams. Magnesium is reacted with oxygen from the air in a crucible, and the masses before and after the oxidation are measured. Magnesium oxide can be made by heating magnesium metal in the presence of oxygen. For example, in the reaction of magnesium metal and oxygen, calculate the mass of magnesium oxide that can be. Magnesium Oxide Theoretical Yield.
From www.bartleby.com
Answered (ii) Magnesium reacts with oxygen to… bartleby Magnesium Oxide Theoretical Yield Magnesium is reacted with oxygen from the air in a crucible, and the masses before and after the oxidation are measured. \(\ce{mgo}\) is the only product in the reaction. Then we will use the. Also determine the amount of excess reactant. Mgco 3 → mgo + co 2. 2mg (s) + o2(g) → 2mgo. What is the percent yield of. Magnesium Oxide Theoretical Yield.
From www.numerade.com
SOLVED Magnesium oxide can be produced by heating magnesium metal in Magnesium Oxide Theoretical Yield Mgco 3 → mgo + co 2. The maximum amount of product(s) that can be obtained in a reaction from a given amount of reactant(s) is the theoretical yield of the. \(\ce{mgo}\) is the only product in the reaction. The theoretical yield is 19 grams. 2mg (s) + o2(g) → 2mgo. Magnesium oxide can be made by heating magnesium metal. Magnesium Oxide Theoretical Yield.
From ar.inspiredpencil.com
Theoretical Yield Definition Magnesium Oxide Theoretical Yield Also determine the amount of excess reactant. Here, you know the actual yield (15 grams) and the theoretical yield (19 grams), so just plug the values into the formula: Mgco 3 → mgo + co 2. Magnesium is reacted with oxygen from the air in a crucible, and the masses before and after the oxidation are measured. \(\ce{mgo}\) is the. Magnesium Oxide Theoretical Yield.
From www.numerade.com
SOLVED Magnesium burns in air with a dazzling brilliance to produce Magnesium Oxide Theoretical Yield The maximum amount of product(s) that can be obtained in a reaction from a given amount of reactant(s) is the theoretical yield of the. This theoretical yield calculator will answer all the burning questions you have regarding how to calculate the theoretical yield,. Magnesium is reacted with oxygen from the air in a crucible, and the masses before and after. Magnesium Oxide Theoretical Yield.
From www.numerade.com
SOLVED Experiment 7 Empirical Formula Objectives Determine the Magnesium Oxide Theoretical Yield Magnesium is reacted with oxygen from the air in a crucible, and the masses before and after the oxidation are measured. Also determine the amount of excess reactant. Then we will use the. Here, you know the actual yield (15 grams) and the theoretical yield (19 grams), so just plug the values into the formula: \(\ce{mgo}\) is the only product. Magnesium Oxide Theoretical Yield.
From www.numerade.com
VIDEO solution 4. Magnesium is the limiting reactant in this Magnesium Oxide Theoretical Yield Magnesium is reacted with oxygen from the air in a crucible, and the masses before and after the oxidation are measured. This theoretical yield calculator will answer all the burning questions you have regarding how to calculate the theoretical yield,. Magnesium oxide can be made by heating magnesium metal in the presence of oxygen. For example, in the reaction of. Magnesium Oxide Theoretical Yield.
From www.numerade.com
SOLVED Magnesium oxide can be made by heating magnesium metal in the Magnesium Oxide Theoretical Yield This theoretical yield calculator will answer all the burning questions you have regarding how to calculate the theoretical yield,. We will use stoichiometry to determine the theoretical yield of magnesium oxide. For example, in the reaction of magnesium metal and oxygen, calculate the mass of magnesium oxide that can be produced if 2.40 g \(mg\) reacts with 10.0 g \(o_2\).. Magnesium Oxide Theoretical Yield.
From www.numerade.com
SOLVED Magnesium burns in air with dazzling brilliance to produce Magnesium Oxide Theoretical Yield Also determine the amount of excess reactant. What is the percent yield of magnesium oxide? Percent yield = actual yield/theoretical yield x 100% percent yield = 15 g/19 g x 100% percent yield = 79% For example, in the reaction of magnesium metal and oxygen, calculate the mass of magnesium oxide that can be produced if 2.40 g \(mg\) reacts. Magnesium Oxide Theoretical Yield.
From www.numerade.com
SOLVED sample of 5.00 of magnesium hydroxide was added to 485 mL of Magnesium Oxide Theoretical Yield \(\ce{mgo}\) is the only product in the reaction. Then we will use the. This theoretical yield calculator will answer all the burning questions you have regarding how to calculate the theoretical yield,. Here, you know the actual yield (15 grams) and the theoretical yield (19 grams), so just plug the values into the formula: Magnesium is reacted with oxygen from. Magnesium Oxide Theoretical Yield.
From www.numerade.com
SOLVEDMagnesium oxide can be made by heating magnesium metal in the Magnesium Oxide Theoretical Yield \(\ce{mgo}\) is the only product in the reaction. Magnesium is reacted with oxygen from the air in a crucible, and the masses before and after the oxidation are measured. Then we will use the. The theoretical yield is 19 grams. The maximum amount of product(s) that can be obtained in a reaction from a given amount of reactant(s) is the. Magnesium Oxide Theoretical Yield.
From www.numerade.com
SOLVED Magnesium oxide can be made by heating magnesium metal in the Magnesium Oxide Theoretical Yield 2mg (s) + o2(g) → 2mgo. Percent yield = actual yield/theoretical yield x 100% percent yield = 15 g/19 g x 100% percent yield = 79% Here, you know the actual yield (15 grams) and the theoretical yield (19 grams), so just plug the values into the formula: This theoretical yield calculator will answer all the burning questions you have. Magnesium Oxide Theoretical Yield.
From www.numerade.com
SOLVED 43. (8 pts) Consider the following balanced equation Mg N Magnesium Oxide Theoretical Yield This theoretical yield calculator will answer all the burning questions you have regarding how to calculate the theoretical yield,. For example, in the reaction of magnesium metal and oxygen, calculate the mass of magnesium oxide that can be produced if 2.40 g \(mg\) reacts with 10.0 g \(o_2\). The maximum amount of product(s) that can be obtained in a reaction. Magnesium Oxide Theoretical Yield.
From www.youtube.com
Percentage yield of magnesium oxide YouTube Magnesium Oxide Theoretical Yield Then we will use the. We will use stoichiometry to determine the theoretical yield of magnesium oxide. Percent yield = actual yield/theoretical yield x 100% percent yield = 15 g/19 g x 100% percent yield = 79% The maximum amount of product(s) that can be obtained in a reaction from a given amount of reactant(s) is the theoretical yield of. Magnesium Oxide Theoretical Yield.
From www.chegg.com
Solved LAB Empirical formula, Yield of Magnesium Oxide Magnesium Oxide Theoretical Yield We will use stoichiometry to determine the theoretical yield of magnesium oxide. \(\ce{mgo}\) is the only product in the reaction. Then we will use the. Here, you know the actual yield (15 grams) and the theoretical yield (19 grams), so just plug the values into the formula: The maximum amount of product(s) that can be obtained in a reaction from. Magnesium Oxide Theoretical Yield.
From www.chegg.com
Solved 11. Calculate the theoretical yield of magnesium Magnesium Oxide Theoretical Yield Mgco 3 → mgo + co 2. Magnesium is reacted with oxygen from the air in a crucible, and the masses before and after the oxidation are measured. The maximum amount of product(s) that can be obtained in a reaction from a given amount of reactant(s) is the theoretical yield of the. Then we will use the. \(\ce{mgo}\) is the. Magnesium Oxide Theoretical Yield.
From www.numerade.com
SOLVED CHEM 1151L Lab Vanual Rug" Qua mass clean empty crucible after Magnesium Oxide Theoretical Yield Here, you know the actual yield (15 grams) and the theoretical yield (19 grams), so just plug the values into the formula: 2mg (s) + o2(g) → 2mgo. This theoretical yield calculator will answer all the burning questions you have regarding how to calculate the theoretical yield,. Then we will use the. Also determine the amount of excess reactant. We. Magnesium Oxide Theoretical Yield.
From www.wikihow.com
How to Calculate Theoretical Yield 12 Steps (with Pictures) Magnesium Oxide Theoretical Yield \(\ce{mgo}\) is the only product in the reaction. For example, in the reaction of magnesium metal and oxygen, calculate the mass of magnesium oxide that can be produced if 2.40 g \(mg\) reacts with 10.0 g \(o_2\). We will use stoichiometry to determine the theoretical yield of magnesium oxide. This theoretical yield calculator will answer all the burning questions you. Magnesium Oxide Theoretical Yield.
From www.numerade.com
SOLVED 'Experiment 8 QUANTITIES IN CHEMICAL REACTIONS PreLab Magnesium Oxide Theoretical Yield The theoretical yield is 19 grams. \(\ce{mgo}\) is the only product in the reaction. 2mg (s) + o2(g) → 2mgo. Magnesium is reacted with oxygen from the air in a crucible, and the masses before and after the oxidation are measured. Here, you know the actual yield (15 grams) and the theoretical yield (19 grams), so just plug the values. Magnesium Oxide Theoretical Yield.
From complianceportal.american.edu
😝 Magnesium oxide production lab. Magnesium Oxide Experiment Lab Report Magnesium Oxide Theoretical Yield 2mg (s) + o2(g) → 2mgo. We will use stoichiometry to determine the theoretical yield of magnesium oxide. The maximum amount of product(s) that can be obtained in a reaction from a given amount of reactant(s) is the theoretical yield of the. Here, you know the actual yield (15 grams) and the theoretical yield (19 grams), so just plug the. Magnesium Oxide Theoretical Yield.
From www.numerade.com
SOLVED "Find limiting reactant and calculate thc theoretical yield of Magnesium Oxide Theoretical Yield Also determine the amount of excess reactant. What is the percent yield of magnesium oxide? For example, in the reaction of magnesium metal and oxygen, calculate the mass of magnesium oxide that can be produced if 2.40 g \(mg\) reacts with 10.0 g \(o_2\). The theoretical yield is 19 grams. \(\ce{mgo}\) is the only product in the reaction. Magnesium oxide. Magnesium Oxide Theoretical Yield.
From brainly.com
Magnesium is the limiting reactant in this experiment. calculate the Magnesium Oxide Theoretical Yield The theoretical yield is 19 grams. Percent yield = actual yield/theoretical yield x 100% percent yield = 15 g/19 g x 100% percent yield = 79% Here, you know the actual yield (15 grams) and the theoretical yield (19 grams), so just plug the values into the formula: This theoretical yield calculator will answer all the burning questions you have. Magnesium Oxide Theoretical Yield.