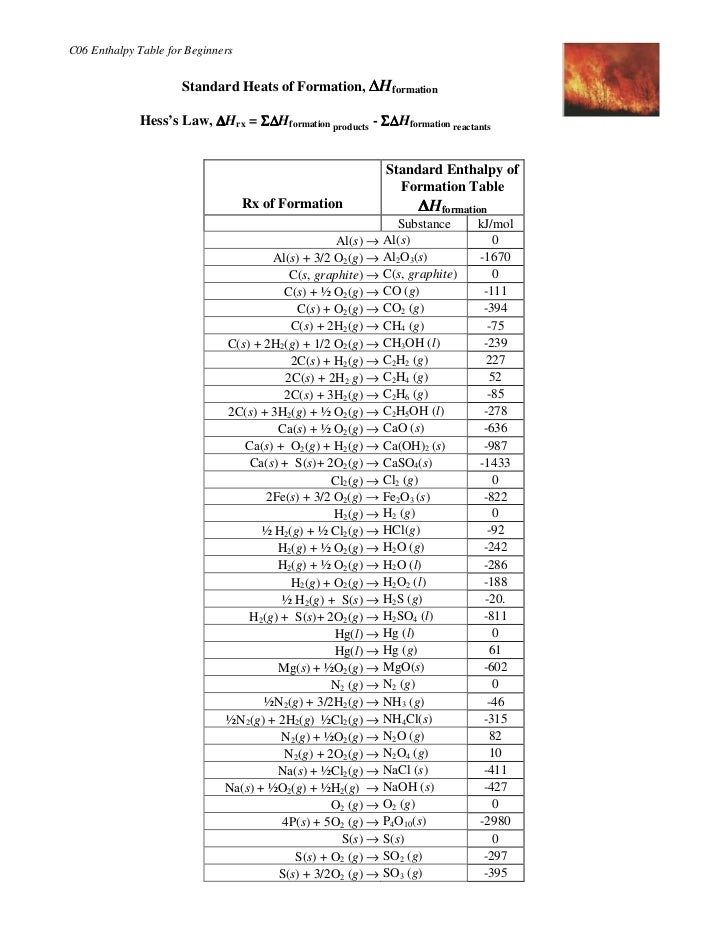
Standard Heat Table . this table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their. 193 rows — in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. standard enthalpies of formation. The standard state heat of formation for the elemental form of each atom is zero. — the elemental form of each atom is that with the lowest enthalpy in the standard state. All standard state, 25 °c and 1 bar (written to 1 decimal place). the table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar. 136 rows — standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements.
from www.slideshare.net
the table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar. All standard state, 25 °c and 1 bar (written to 1 decimal place). a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. 136 rows — standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. this table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their. The standard state heat of formation for the elemental form of each atom is zero. standard enthalpies of formation. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. 193 rows — in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of.
Heat of formation by reactions
Standard Heat Table 136 rows — standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. All standard state, 25 °c and 1 bar (written to 1 decimal place). 193 rows — in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. this table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. — the elemental form of each atom is that with the lowest enthalpy in the standard state. 136 rows — standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar. standard enthalpies of formation. The standard state heat of formation for the elemental form of each atom is zero.
From marielatinrobertson.blogspot.com
Standard Enthalpy of Combustion MarielatinRobertson Standard Heat Table The standard state heat of formation for the elemental form of each atom is zero. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. — the elemental form of each atom is that with the lowest enthalpy in the standard state. standard enthalpies of formation. All standard state,. Standard Heat Table.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all Standard Heat Table The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. 193 rows — in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. — the elemental form of each atom is that with the lowest enthalpy in the standard state. The standard state heat. Standard Heat Table.
From rayb78.github.io
Heat Of Formation Chart Standard Heat Table the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. 193 rows — in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. The standard state heat of formation for the elemental form of each atom is zero. — the. Standard Heat Table.
From exokycsnc.blob.core.windows.net
Standard Enthalpy Of Formation Table Elements at Filomena Gilbert blog Standard Heat Table 136 rows — standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which. Standard Heat Table.
From exoyndeil.blob.core.windows.net
Standard Enthalpy Of Formation In Elements at Michael Zapien blog Standard Heat Table this table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their. the table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements.. Standard Heat Table.
From www.slideserve.com
PPT Chapter 5 Thermochemistry PowerPoint Presentation, free download Standard Heat Table The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. this table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements. Standard Heat Table.
From stahonorschemistry.weebly.com
III Calculating Enthalpies STA Form IV Honors Chemistry Standard Heat Table this table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their. 136 rows — standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole. Standard Heat Table.
From www.semanticscholar.org
Table I from Largescale calculations of gas phase thermochemistry Standard Heat Table a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. 193 rows — in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. this table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from. Standard Heat Table.
From heatindexinrimu.blogspot.com
Heat Index Osha Heat Index Chart Standard Heat Table — the elemental form of each atom is that with the lowest enthalpy in the standard state. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. 136 rows — standard enthalpy change of formation (data table) these tables include heat of formation data gathered. Standard Heat Table.
From exokycsnc.blob.core.windows.net
Standard Enthalpy Of Formation Table Elements at Filomena Gilbert blog Standard Heat Table The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. 136 rows — standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. — the elemental form of each atom is that with the lowest enthalpy in the standard state. the. Standard Heat Table.
From www.electricalelibrary.com
What is thermal perception? Electrical Standard Heat Table 136 rows — standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. 193 rows — in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. — the elemental form of each atom is that with the lowest enthalpy in the standard state. this. Standard Heat Table.
From exoziqnph.blob.core.windows.net
Standard Heat Of Formation Co2 at Jonathan Blackburn blog Standard Heat Table The standard state heat of formation for the elemental form of each atom is zero. the table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. All standard state, 25 °c. Standard Heat Table.
From lessonluft.z19.web.core.windows.net
Heat Of Formation Chart Standard Heat Table a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. this table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their. 193 rows — in chemistry and thermodynamics, the standard enthalpy of formation or standard heat. Standard Heat Table.
From www.coursehero.com
[Solved] . From the data in the tables, calculate the heat of Standard Heat Table All standard state, 25 °c and 1 bar (written to 1 decimal place). — the elemental form of each atom is that with the lowest enthalpy in the standard state. 136 rows — standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. this table lists the standard enthalpies (δh°),. Standard Heat Table.
From www.slideshare.net
Heat of formation by reactions Standard Heat Table The standard state heat of formation for the elemental form of each atom is zero. 136 rows — standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. 193 rows — in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. — the elemental form. Standard Heat Table.
From www.scribd.com
Standard Thermodynamic Values Formula State of Matter Enthalpy (kJ/mol Standard Heat Table the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar. 136 rows — standard enthalpy change of formation (data table) these tables include heat of. Standard Heat Table.
From wisataparatravell.blogspot.com
Standard Heats Of Formation Standard Enthalpies Of Formation And The Standard Heat Table 136 rows — standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. The standard state heat of formation for the elemental form of each atom is zero. standard enthalpies of formation. the table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy. Standard Heat Table.
From www.goconqr.com
DF4 Mapa Mental Standard Heat Table the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. The standard state heat of formation for the elemental form of each atom is zero. All standard state, 25 °c and 1 bar (written to 1 decimal place). The enthalpy of formation (\(δh_{f}\)) is the enthalpy change. Standard Heat Table.
From www.studocu.com
Standard Enthalpies of Formation & Standard Entropies kJ J ( mol Standard Heat Table The standard state heat of formation for the elemental form of each atom is zero. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. All standard state, 25 °c and 1 bar (written to 1 decimal place). this table lists the standard enthalpies (δh°),. Standard Heat Table.
From z-cm.blogspot.com
How To Use Thermodynamic Tables Decoration Examples Standard Heat Table — the elemental form of each atom is that with the lowest enthalpy in the standard state. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. All standard state, 25 °c and 1 bar (written to 1 decimal place). the table below shows the standard enthalpy of formation,. Standard Heat Table.
From mavink.com
Standard Enthalpy Chart Standard Heat Table standard enthalpies of formation. — the elemental form of each atom is that with the lowest enthalpy in the standard state. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. this table lists the standard enthalpies (δh°), the free energies (δg°) of formation. Standard Heat Table.
From chemistry.stackexchange.com
physical chemistry Why is the standard enthalpy of formation Standard Heat Table the table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. All standard state, 25 °c and 1 bar (written to 1 decimal place). —. Standard Heat Table.
From www.researchgate.net
Thermodynamic data enthalpy and heat capacity 21,22 . Download Table Standard Heat Table standard enthalpies of formation. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the table below shows the standard enthalpy of formation, the standard gibbs free energy of formation, standard entropy and molar. — the elemental form of each atom is that with. Standard Heat Table.
From exosbjzsg.blob.core.windows.net
Standard Enthalpy Of Formation Al2O3 at Lois Woodburn blog Standard Heat Table a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. this table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their. 136 rows — standard enthalpy change of formation (data table) these tables include heat of. Standard Heat Table.
From mungfali.com
Standard Thermodynamic Table Standard Heat Table a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. All standard state, 25 °c and 1 bar (written to 1 decimal place). — the elemental form of each atom is that with the lowest enthalpy in the standard state. the table below shows. Standard Heat Table.
From darkataxa.blogspot.com
Astounding Collections Of Heat Of Formation Table Photos Darkata Standard Heat Table the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. All standard state, 25 °c and 1 bar (written to 1 decimal place). standard enthalpies of formation. this table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in. Standard Heat Table.
From duanerafanan.blogspot.com
DUANE HESS'S LAW Standard Heat Table 136 rows — standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. All standard state, 25 °c and 1 bar (written to 1 decimal place). 193 rows — in chemistry and. Standard Heat Table.
From ar.inspiredpencil.com
Heat Of Formation Table Standard Heat Table The standard state heat of formation for the elemental form of each atom is zero. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. All standard state, 25 °c and 1 bar (written to 1 decimal place). — the elemental form of each atom is that with the lowest. Standard Heat Table.
From exokycsnc.blob.core.windows.net
Standard Enthalpy Of Formation Table Elements at Filomena Gilbert blog Standard Heat Table The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. — the elemental form of each atom is that with the lowest enthalpy in the standard state. 136 rows — standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. the. Standard Heat Table.
From mavink.com
Types Of Heat Exchangers Standard Heat Table 136 rows — standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. this table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their. 193 rows — in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of.. Standard Heat Table.
From www.chegg.com
Solved Appendix C Thermodynamic Data Table C.1 Standard E... Standard Heat Table The standard state heat of formation for the elemental form of each atom is zero. this table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their. 136 rows — standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. — the elemental. Standard Heat Table.
From www.studocu.com
Standard Enthalpy of Formation Table Standard Enthalpy of Formation Standard Heat Table All standard state, 25 °c and 1 bar (written to 1 decimal place). the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. The standard state heat of formation for the elemental form of each atom is zero. 136 rows — standard enthalpy change of formation. Standard Heat Table.
From exoluenrv.blob.core.windows.net
Standard Enthalpy Of Formation Diamond at James Torres blog Standard Heat Table this table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their. All standard state, 25 °c and 1 bar (written to 1 decimal place). 136 rows — standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. The standard state heat of formation. Standard Heat Table.
From exokycsnc.blob.core.windows.net
Standard Enthalpy Of Formation Table Elements at Filomena Gilbert blog Standard Heat Table this table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their. All standard state, 25 °c and 1 bar (written to 1 decimal place). a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. The enthalpy of. Standard Heat Table.
From www.semanticscholar.org
[PDF] Largescale calculations of gas phase thermochemistry Enthalpy Standard Heat Table a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. 136 rows — standard enthalpy change of formation (data table) these tables. Standard Heat Table.