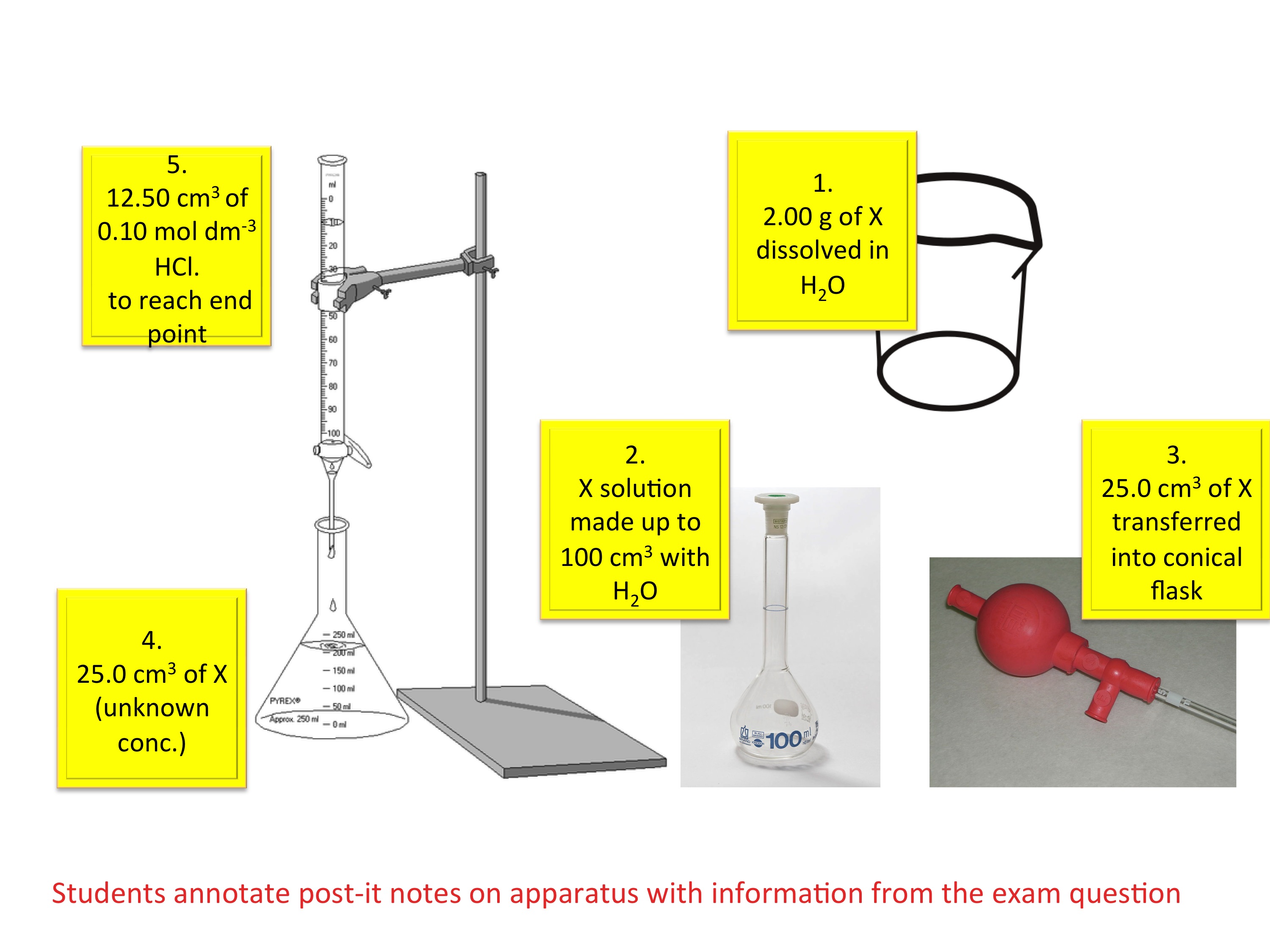
Steps For A Titration . A measured volume of an acid of unknown concentration is added to an erlenmeyer flask. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The steps in a titration reaction are outlined below. Learn what titration is, how to use indicators like starch and phenolphthalein, and how to calculate the amount of titrand in a solution. In our example we would accurately measure a known. Several drops of an indicator are added to the. The first step is to measure out a known volume of the sample that you want to find the concentration of into a flask. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with another. Titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction occurs.
from mungfali.com
A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. Titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction occurs. Learn what titration is, how to use indicators like starch and phenolphthalein, and how to calculate the amount of titrand in a solution. The first step is to measure out a known volume of the sample that you want to find the concentration of into a flask. Several drops of an indicator are added to the. In our example we would accurately measure a known. A measured volume of an acid of unknown concentration is added to an erlenmeyer flask. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with another. The steps in a titration reaction are outlined below.
Titration Steps
Steps For A Titration A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Learn what titration is, how to use indicators like starch and phenolphthalein, and how to calculate the amount of titrand in a solution. Several drops of an indicator are added to the. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. A measured volume of an acid of unknown concentration is added to an erlenmeyer flask. The steps in a titration reaction are outlined below. The first step is to measure out a known volume of the sample that you want to find the concentration of into a flask. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with another. In our example we would accurately measure a known. Titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction occurs. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.
From www.slideserve.com
PPT Neutralization & Titrations PowerPoint Presentation, free Steps For A Titration The steps in a titration reaction are outlined below. Titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction occurs. Several drops of an indicator are added to the. Learn what titration is, how to use indicators like starch and phenolphthalein, and how to calculate the amount of titrand in. Steps For A Titration.
From www.compoundchem.com
Chemistry Techniques Titration Compound Interest Steps For A Titration The steps in a titration reaction are outlined below. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with another. Several drops of an indicator are added to the. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.. Steps For A Titration.
From www.slideserve.com
PPT 8.5 AcidBase Reactions PowerPoint Presentation, free download Steps For A Titration Titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction occurs. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with another. Several drops of an indicator are added to the. A titration is a laboratory technique used to. Steps For A Titration.
From theedge.com.hk
Chemistry How To Titration The Edge Steps For A Titration The steps in a titration reaction are outlined below. A measured volume of an acid of unknown concentration is added to an erlenmeyer flask. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. Titration is a technique to. Steps For A Titration.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download ID Steps For A Titration A measured volume of an acid of unknown concentration is added to an erlenmeyer flask. In our example we would accurately measure a known. Learn what titration is, how to use indicators like starch and phenolphthalein, and how to calculate the amount of titrand in a solution. The first step is to measure out a known volume of the sample. Steps For A Titration.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube Steps For A Titration A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. The first step is to measure out a known volume of the sample that you want to find the concentration of into a flask. 1.1 a titration involves measuring. Steps For A Titration.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Steps For A Titration The steps in a titration reaction are outlined below. A measured volume of an acid of unknown concentration is added to an erlenmeyer flask. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. The first step is to. Steps For A Titration.
From www.slideserve.com
PPT ENZYME CATALYSIS LAB PowerPoint Presentation ID5750147 Steps For A Titration A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. The steps in a titration reaction are outlined below. A measured volume of an acid of unknown concentration is added to an erlenmeyer flask. Several drops of an indicator. Steps For A Titration.
From mungfali.com
Acid Base Titration Procedure Steps For A Titration A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with another. Several drops of an indicator are added to the. A measured volume of an acid of unknown concentration. Steps For A Titration.
From mungfali.com
Titration Steps Steps For A Titration A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A measured volume of an acid of unknown concentration is added to an erlenmeyer flask. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with another. In our example. Steps For A Titration.
From www.youtube.com
Titration Calculations AQA GCSE Chemistry YouTube Steps For A Titration 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with another. Learn what titration is, how to use indicators like starch and phenolphthalein, and how to calculate the amount of titrand in a solution. The first step is to measure out a known volume of the sample that you. Steps For A Titration.
From general.chemistrysteps.com
Titration of a Polyprotic Acids Chemistry Steps Steps For A Titration Several drops of an indicator are added to the. The steps in a titration reaction are outlined below. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction occurs. 1.1. Steps For A Titration.
From www.slideserve.com
PPT AcidBase Titration and pH PowerPoint Presentation, free download Steps For A Titration A measured volume of an acid of unknown concentration is added to an erlenmeyer flask. The steps in a titration reaction are outlined below. Titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction occurs. In our example we would accurately measure a known. 1.1 a titration involves measuring the. Steps For A Titration.
From mungfali.com
Titration Steps Steps For A Titration A measured volume of an acid of unknown concentration is added to an erlenmeyer flask. Learn what titration is, how to use indicators like starch and phenolphthalein, and how to calculate the amount of titrand in a solution. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. In our. Steps For A Titration.
From www.slideserve.com
PPT AcidBase Titration and pH PowerPoint Presentation, free download Steps For A Titration In our example we would accurately measure a known. The first step is to measure out a known volume of the sample that you want to find the concentration of into a flask. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until. Steps For A Titration.
From labthink.netlify.app
What is titration and how does it work Steps For A Titration Learn what titration is, how to use indicators like starch and phenolphthalein, and how to calculate the amount of titrand in a solution. Several drops of an indicator are added to the. A measured volume of an acid of unknown concentration is added to an erlenmeyer flask. In our example we would accurately measure a known. A titration is a. Steps For A Titration.
From www.analytica-world.com
Titration Handbook Theory and Practice of Titration A guide to Steps For A Titration 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with another. Learn what titration is, how to use indicators like starch and phenolphthalein, and how to calculate the amount of titrand in a solution. Titration is a technique to determine the concentration of an unknown solution by adding a. Steps For A Titration.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators Steps For A Titration Learn what titration is, how to use indicators like starch and phenolphthalein, and how to calculate the amount of titrand in a solution. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is a technique to determine the concentration of an unknown solution by adding a known solution. Steps For A Titration.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Steps For A Titration A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. The steps in a titration reaction are outlined below. A measured volume of an acid of unknown concentration is added to an erlenmeyer flask. In our example we would. Steps For A Titration.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Steps For A Titration Learn what titration is, how to use indicators like starch and phenolphthalein, and how to calculate the amount of titrand in a solution. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is a technique to determine the concentration of an unknown solution by adding a known solution. Steps For A Titration.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Steps For A Titration The steps in a titration reaction are outlined below. Several drops of an indicator are added to the. The first step is to measure out a known volume of the sample that you want to find the concentration of into a flask. Titration is a technique to determine the concentration of an unknown solution by adding a known solution until. Steps For A Titration.
From www.slideserve.com
PPT Neutralization & Titrations PowerPoint Presentation, free Steps For A Titration Learn what titration is, how to use indicators like starch and phenolphthalein, and how to calculate the amount of titrand in a solution. In our example we would accurately measure a known. Titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction occurs. The first step is to measure out. Steps For A Titration.
From www.savemyexams.co.uk
Titrations (1.2.9) DP IB Chemistry SL Revision Notes 2016 Save My Steps For A Titration A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Learn what titration is, how to use indicators like. Steps For A Titration.
From www.slideserve.com
PPT AcidBase Titration and pH PowerPoint Presentation, free download Steps For A Titration The first step is to measure out a known volume of the sample that you want to find the concentration of into a flask. A measured volume of an acid of unknown concentration is added to an erlenmeyer flask. In our example we would accurately measure a known. Learn what titration is, how to use indicators like starch and phenolphthalein,. Steps For A Titration.
From www.youtube.com
Titration procedure (Step by step) YouTube Steps For A Titration In our example we would accurately measure a known. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. A measured volume of an acid of unknown concentration is added to an erlenmeyer flask. Titration is a technique to. Steps For A Titration.
From general.chemistrysteps.com
Titration of a Weak Acid by a Strong Base Chemistry Steps Steps For A Titration In our example we would accurately measure a known. The steps in a titration reaction are outlined below. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a. Steps For A Titration.
From www.slideserve.com
PPT AcidBase Titration and pH PowerPoint Presentation, free download Steps For A Titration A measured volume of an acid of unknown concentration is added to an erlenmeyer flask. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with another. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Several drops of. Steps For A Titration.
From www.tes.com
Titration Edexcel 91 Separate (Triple) Science Teaching Resources Steps For A Titration The steps in a titration reaction are outlined below. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with another. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence. Steps For A Titration.
From letitsnowglobe.co.uk
Titration procedure pdf Steps For A Titration In our example we would accurately measure a known. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely. Steps For A Titration.
From www.science-revision.co.uk
Titrations Steps For A Titration Titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction occurs. In our example we would accurately measure a known. Several drops of an indicator are added to the. A measured volume of an acid of unknown concentration is added to an erlenmeyer flask. The first step is to measure. Steps For A Titration.
From letitsnowglobe.co.uk
Titration procedure pdf Steps For A Titration Several drops of an indicator are added to the. Titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction occurs. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point. Steps For A Titration.
From www.youtube.com
Titration How to perform Titration Experiment/Practical YouTube Steps For A Titration Several drops of an indicator are added to the. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. A measured volume of an acid of unknown concentration is added to an erlenmeyer flask. The steps in a titration. Steps For A Titration.
From www.tutormyself.com
233 (Triple only) describe how to carry out an acidalkali titration Steps For A Titration Titration is a technique to determine the concentration of an unknown solution by adding a known solution until a reaction occurs. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A measured volume of an acid of unknown concentration is added to an erlenmeyer flask. In our example we. Steps For A Titration.
From www.slideserve.com
PPT Titrations PowerPoint Presentation, free download ID2145164 Steps For A Titration A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The first step is to measure out a known volume of the sample that you want to find the concentration of into a flask. In our example we would accurately measure a known. The steps in a titration reaction are. Steps For A Titration.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Steps For A Titration A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The first step is to measure out a known volume of the sample that you want to find the concentration of into a flask. In our example we would accurately measure a known. 1.1 a titration involves measuring the exact. Steps For A Titration.