An Endothermic Solution Process Is Described By Which Of The Following . B) δh > 0, solution feels hot. Change in heat is greater than 0, solution feels cold. An exothermic solution process is described by which of the following?multiple choice. Heat energy is absorbed from the pan to cook the egg. C) δh < 0, solution feels cold. Click the card to flip 👆. Click the card to flip 👆. chem 212 connect practice quiz. The quantity of heat for a process is represented by the letter q q. when a solvent is added to a solution, steps 1 and 2 are both endothermic because energy is required to overcome the intermolecular interactions in the solvent ( δh1) and the solute ( δh2 ). The opposite of an endothermic. The first law of thermodynamics. an endothermic solution process is described by which of the following? A) δh > 0, solution feels cold. Click the card to flip 👆.
from www.worksheetsplanet.com
B) δh > 0, solution feels hot. Click the card to flip 👆. A) δh > 0, solution feels cold. Change in heat is greater than 0, solution feels cold. an endothermic process may be a chemical process, such as dissolving ammonium nitrate ( nh4no3) in water ( h2o ), or a physical process, such as the melting of ice cubes. C) δh < 0, solution feels cold. Heat energy is absorbed from the pan to cook the egg. An exothermic solution process is described by which of the following?multiple choice. chem 212 connect practice quiz. in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases.
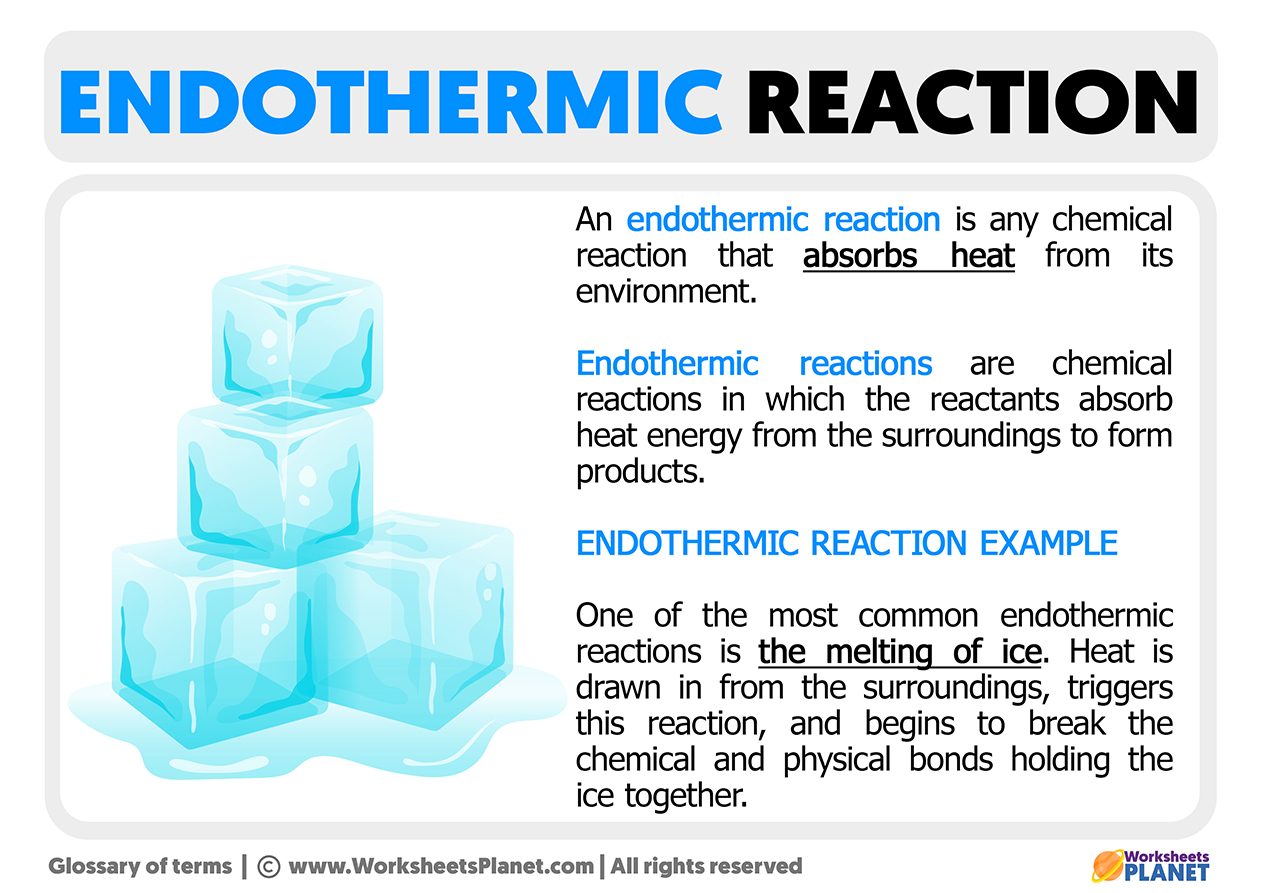
What is an Endothermic Reaction Definition & Example
An Endothermic Solution Process Is Described By Which Of The Following when a solvent is added to a solution, steps 1 and 2 are both endothermic because energy is required to overcome the intermolecular interactions in the solvent ( δh1) and the solute ( δh2 ). an endothermic solution process is described by which of the following? in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. when a solvent is added to a solution, steps 1 and 2 are both endothermic because energy is required to overcome the intermolecular interactions in the solvent ( δh1) and the solute ( δh2 ). The opposite of an endothermic. chem 212 connect practice quiz. Click the card to flip 👆. Heat energy is absorbed from the pan to cook the egg. A) δh > 0, solution feels cold. Click the card to flip 👆. B) δh > 0, solution feels hot. Change in heat is greater than 0, solution feels cold. An exothermic solution process is described by which of the following?multiple choice. The first law of thermodynamics. C) δh < 0, solution feels cold. The quantity of heat for a process is represented by the letter q q.
From www.chegg.com
Solved Which of the following processes is endothermic? An An Endothermic Solution Process Is Described By Which Of The Following Click the card to flip 👆. C) δh < 0, solution feels cold. Plants absorb heat energy from sunlight to convert carbon dioxide and water into glucose and oxygen. Change in heat is greater than 0, solution feels cold. an endothermic process may be a chemical process, such as dissolving ammonium nitrate ( nh4no3) in water ( h2o ),. An Endothermic Solution Process Is Described By Which Of The Following.
From www.bigstockphoto.com
Endothermic Exothermic Image & Photo (Free Trial) Bigstock An Endothermic Solution Process Is Described By Which Of The Following Change in heat is greater than 0, solution feels cold. chem 212 connect practice quiz. Heat energy is absorbed from the pan to cook the egg. Plants absorb heat energy from sunlight to convert carbon dioxide and water into glucose and oxygen. in the course of an endothermic process, the system gains heat from the surroundings and so. An Endothermic Solution Process Is Described By Which Of The Following.
From www.flexiprep.com
NCERT Class X Science Class Chapter 1. Chemical Reactions and An Endothermic Solution Process Is Described By Which Of The Following A) δh > 0, solution feels cold. C) δh < 0, solution feels cold. Change in heat is greater than 0, solution feels cold. Heat energy is absorbed from the pan to cook the egg. The first law of thermodynamics. an endothermic process may be a chemical process, such as dissolving ammonium nitrate ( nh4no3) in water ( h2o. An Endothermic Solution Process Is Described By Which Of The Following.
From byjus.com
Difference Between Endothermic and Exothermic Reactions Chemistry An Endothermic Solution Process Is Described By Which Of The Following an endothermic process may be a chemical process, such as dissolving ammonium nitrate ( nh4no3) in water ( h2o ), or a physical process, such as the melting of ice cubes. C) δh < 0, solution feels cold. B) δh > 0, solution feels hot. an endothermic solution process is described by which of the following? The first. An Endothermic Solution Process Is Described By Which Of The Following.
From www.numerade.com
SOLVEDFrom the statements below, say whether the following processes An Endothermic Solution Process Is Described By Which Of The Following in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. The first law of thermodynamics. C) δh < 0, solution feels cold. The opposite of an endothermic. Plants absorb heat energy from sunlight to convert carbon dioxide and water into glucose and oxygen. Click the card to. An Endothermic Solution Process Is Described By Which Of The Following.
From www.slideserve.com
PPT Solutions and Their Properties PowerPoint Presentation, free An Endothermic Solution Process Is Described By Which Of The Following Plants absorb heat energy from sunlight to convert carbon dioxide and water into glucose and oxygen. when a solvent is added to a solution, steps 1 and 2 are both endothermic because energy is required to overcome the intermolecular interactions in the solvent ( δh1) and the solute ( δh2 ). Heat energy is absorbed from the pan to. An Endothermic Solution Process Is Described By Which Of The Following.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples An Endothermic Solution Process Is Described By Which Of The Following B) δh > 0, solution feels hot. Plants absorb heat energy from sunlight to convert carbon dioxide and water into glucose and oxygen. chem 212 connect practice quiz. The opposite of an endothermic. an endothermic process may be a chemical process, such as dissolving ammonium nitrate ( nh4no3) in water ( h2o ), or a physical process, such. An Endothermic Solution Process Is Described By Which Of The Following.
From classnotes123.com
What does one mean by exothermic and endothermic reactions? Give An Endothermic Solution Process Is Described By Which Of The Following The opposite of an endothermic. when a solvent is added to a solution, steps 1 and 2 are both endothermic because energy is required to overcome the intermolecular interactions in the solvent ( δh1) and the solute ( δh2 ). Click the card to flip 👆. in the course of an endothermic process, the system gains heat from. An Endothermic Solution Process Is Described By Which Of The Following.
From letstalkscience.ca
The Cold Pack A Chilly Example of an Endothermic Reaction Let's Talk An Endothermic Solution Process Is Described By Which Of The Following A) δh > 0, solution feels cold. Plants absorb heat energy from sunlight to convert carbon dioxide and water into glucose and oxygen. C) δh < 0, solution feels cold. The quantity of heat for a process is represented by the letter q q. The first law of thermodynamics. an endothermic solution process is described by which of the. An Endothermic Solution Process Is Described By Which Of The Following.
From www.chegg.com
Solved Part A Identify whether each process is endothermic An Endothermic Solution Process Is Described By Which Of The Following Click the card to flip 👆. The quantity of heat for a process is represented by the letter q q. an endothermic solution process is described by which of the following? an endothermic process may be a chemical process, such as dissolving ammonium nitrate ( nh4no3) in water ( h2o ), or a physical process, such as the. An Endothermic Solution Process Is Described By Which Of The Following.
From www.youtube.com
Endothermic and exothermic reactions. Enthalpy YouTube An Endothermic Solution Process Is Described By Which Of The Following The quantity of heat for a process is represented by the letter q q. B) δh > 0, solution feels hot. Heat energy is absorbed from the pan to cook the egg. an endothermic solution process is described by which of the following? when a solvent is added to a solution, steps 1 and 2 are both endothermic. An Endothermic Solution Process Is Described By Which Of The Following.
From www.toppr.com
Give an example each of an exothermic an endothermic process. An Endothermic Solution Process Is Described By Which Of The Following A) δh > 0, solution feels cold. C) δh < 0, solution feels cold. The first law of thermodynamics. Heat energy is absorbed from the pan to cook the egg. chem 212 connect practice quiz. Change in heat is greater than 0, solution feels cold. Plants absorb heat energy from sunlight to convert carbon dioxide and water into glucose. An Endothermic Solution Process Is Described By Which Of The Following.
From www.chegg.com
Chemistry Archive March 06, 2020 An Endothermic Solution Process Is Described By Which Of The Following The opposite of an endothermic. Click the card to flip 👆. Click the card to flip 👆. A) δh > 0, solution feels cold. Plants absorb heat energy from sunlight to convert carbon dioxide and water into glucose and oxygen. an endothermic solution process is described by which of the following? Heat energy is absorbed from the pan to. An Endothermic Solution Process Is Described By Which Of The Following.
From www.doubtnut.com
Doubt Solutions Maths, Science, CBSE, NCERT, IIT JEE, NEET An Endothermic Solution Process Is Described By Which Of The Following an endothermic solution process is described by which of the following? in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. Plants absorb heat energy from sunlight to convert carbon dioxide and water into glucose and oxygen. C) δh < 0, solution feels cold. Click the. An Endothermic Solution Process Is Described By Which Of The Following.
From www.youtube.com
Why are some solution processes exothermic whereas others are An Endothermic Solution Process Is Described By Which Of The Following an endothermic process may be a chemical process, such as dissolving ammonium nitrate ( nh4no3) in water ( h2o ), or a physical process, such as the melting of ice cubes. when a solvent is added to a solution, steps 1 and 2 are both endothermic because energy is required to overcome the intermolecular interactions in the solvent. An Endothermic Solution Process Is Described By Which Of The Following.
From stock.adobe.com
Activation energy in endothermic and exothermic reactions. Stock An Endothermic Solution Process Is Described By Which Of The Following in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. B) δh > 0, solution feels hot. Heat energy is absorbed from the pan to cook the egg. The quantity of heat for a process is represented by the letter q q. an endothermic process may. An Endothermic Solution Process Is Described By Which Of The Following.
From www.worksheetsplanet.com
What is an Endothermic Reaction Definition & Example An Endothermic Solution Process Is Described By Which Of The Following The first law of thermodynamics. Heat energy is absorbed from the pan to cook the egg. C) δh < 0, solution feels cold. Plants absorb heat energy from sunlight to convert carbon dioxide and water into glucose and oxygen. an endothermic solution process is described by which of the following? The quantity of heat for a process is represented. An Endothermic Solution Process Is Described By Which Of The Following.
From www.thoughtco.com
Endothermic Reaction Examples An Endothermic Solution Process Is Described By Which Of The Following B) δh > 0, solution feels hot. Click the card to flip 👆. The quantity of heat for a process is represented by the letter q q. when a solvent is added to a solution, steps 1 and 2 are both endothermic because energy is required to overcome the intermolecular interactions in the solvent ( δh1) and the solute. An Endothermic Solution Process Is Described By Which Of The Following.
From www.chegg.com
An endothermic solution is described by which of the An Endothermic Solution Process Is Described By Which Of The Following B) δh > 0, solution feels hot. when a solvent is added to a solution, steps 1 and 2 are both endothermic because energy is required to overcome the intermolecular interactions in the solvent ( δh1) and the solute ( δh2 ). The opposite of an endothermic. Click the card to flip 👆. an endothermic solution process is. An Endothermic Solution Process Is Described By Which Of The Following.
From www.pinterest.com
Understanding Endothermic and Exothermic Reactions Exothermic An Endothermic Solution Process Is Described By Which Of The Following in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. An exothermic solution process is described by which of the following?multiple choice. Heat energy is absorbed from the pan to cook the egg. an endothermic solution process is described by which of the following? when. An Endothermic Solution Process Is Described By Which Of The Following.
From muadacsan3mien.com
What Are Exothermic And Endothermic Changes? Examples Unveiled! An Endothermic Solution Process Is Described By Which Of The Following The first law of thermodynamics. chem 212 connect practice quiz. an endothermic process may be a chemical process, such as dissolving ammonium nitrate ( nh4no3) in water ( h2o ), or a physical process, such as the melting of ice cubes. an endothermic solution process is described by which of the following? C) δh < 0, solution. An Endothermic Solution Process Is Described By Which Of The Following.
From pressbooks.online.ucf.edu
12.1 The Dissolution Process Chemistry Fundamentals An Endothermic Solution Process Is Described By Which Of The Following The first law of thermodynamics. The quantity of heat for a process is represented by the letter q q. C) δh < 0, solution feels cold. The opposite of an endothermic. An exothermic solution process is described by which of the following?multiple choice. Plants absorb heat energy from sunlight to convert carbon dioxide and water into glucose and oxygen. . An Endothermic Solution Process Is Described By Which Of The Following.
From www.chegg.com
Solved LAn endothermic solution process is described by An Endothermic Solution Process Is Described By Which Of The Following The quantity of heat for a process is represented by the letter q q. Plants absorb heat energy from sunlight to convert carbon dioxide and water into glucose and oxygen. Click the card to flip 👆. B) δh > 0, solution feels hot. An exothermic solution process is described by which of the following?multiple choice. an endothermic process may. An Endothermic Solution Process Is Described By Which Of The Following.
From www.myxxgirl.com
Reaction Energy Diagram Exothermic Diagram Media My XXX Hot Girl An Endothermic Solution Process Is Described By Which Of The Following an endothermic solution process is described by which of the following? Click the card to flip 👆. chem 212 connect practice quiz. B) δh > 0, solution feels hot. An exothermic solution process is described by which of the following?multiple choice. in the course of an endothermic process, the system gains heat from the surroundings and so. An Endothermic Solution Process Is Described By Which Of The Following.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE An Endothermic Solution Process Is Described By Which Of The Following Click the card to flip 👆. The opposite of an endothermic. an endothermic solution process is described by which of the following? Change in heat is greater than 0, solution feels cold. An exothermic solution process is described by which of the following?multiple choice. when a solvent is added to a solution, steps 1 and 2 are both. An Endothermic Solution Process Is Described By Which Of The Following.
From www.chegg.com
Solved Which of the following processes is endothermic?. An Endothermic Solution Process Is Described By Which Of The Following an endothermic solution process is described by which of the following? Click the card to flip 👆. Plants absorb heat energy from sunlight to convert carbon dioxide and water into glucose and oxygen. An exothermic solution process is described by which of the following?multiple choice. Change in heat is greater than 0, solution feels cold. in the course. An Endothermic Solution Process Is Described By Which Of The Following.
From www.chegg.com
Solved Which of the following processes is endothermic? An Endothermic Solution Process Is Described By Which Of The Following an endothermic solution process is described by which of the following? An exothermic solution process is described by which of the following?multiple choice. The first law of thermodynamics. A) δh > 0, solution feels cold. C) δh < 0, solution feels cold. an endothermic process may be a chemical process, such as dissolving ammonium nitrate ( nh4no3) in. An Endothermic Solution Process Is Described By Which Of The Following.
From brainly.com
Classify the following statements/ pictures as either an endothermic or An Endothermic Solution Process Is Described By Which Of The Following The quantity of heat for a process is represented by the letter q q. when a solvent is added to a solution, steps 1 and 2 are both endothermic because energy is required to overcome the intermolecular interactions in the solvent ( δh1) and the solute ( δh2 ). B) δh > 0, solution feels hot. An exothermic solution. An Endothermic Solution Process Is Described By Which Of The Following.
From www.slideserve.com
PPT Endothermic and exothermic reactions PowerPoint Presentation An Endothermic Solution Process Is Described By Which Of The Following Change in heat is greater than 0, solution feels cold. chem 212 connect practice quiz. The first law of thermodynamics. Click the card to flip 👆. Plants absorb heat energy from sunlight to convert carbon dioxide and water into glucose and oxygen. Click the card to flip 👆. An exothermic solution process is described by which of the following?multiple. An Endothermic Solution Process Is Described By Which Of The Following.
From h-o-m-e.org
Chilling with Endothermic Reactions An Endothermic Solution Process Is Described By Which Of The Following The first law of thermodynamics. The opposite of an endothermic. Change in heat is greater than 0, solution feels cold. Click the card to flip 👆. A) δh > 0, solution feels cold. Click the card to flip 👆. when a solvent is added to a solution, steps 1 and 2 are both endothermic because energy is required to. An Endothermic Solution Process Is Described By Which Of The Following.
From study.com
Endothermic vs. Exothermic Reactions Process & Examples Video An Endothermic Solution Process Is Described By Which Of The Following Click the card to flip 👆. The first law of thermodynamics. Heat energy is absorbed from the pan to cook the egg. an endothermic solution process is described by which of the following? A) δh > 0, solution feels cold. An exothermic solution process is described by which of the following?multiple choice. Plants absorb heat energy from sunlight to. An Endothermic Solution Process Is Described By Which Of The Following.
From shaunmwilliams.com
Chapter 11 Presentation An Endothermic Solution Process Is Described By Which Of The Following A) δh > 0, solution feels cold. The quantity of heat for a process is represented by the letter q q. Click the card to flip 👆. Click the card to flip 👆. chem 212 connect practice quiz. The first law of thermodynamics. B) δh > 0, solution feels hot. in the course of an endothermic process, the. An Endothermic Solution Process Is Described By Which Of The Following.
From chem.libretexts.org
13.1 Factors Affecting Solution Formation Chemistry LibreTexts An Endothermic Solution Process Is Described By Which Of The Following B) δh > 0, solution feels hot. an endothermic solution process is described by which of the following? The opposite of an endothermic. an endothermic process may be a chemical process, such as dissolving ammonium nitrate ( nh4no3) in water ( h2o ), or a physical process, such as the melting of ice cubes. Click the card to. An Endothermic Solution Process Is Described By Which Of The Following.
From solvedlib.com
D) Determine if the following processes are endotherm… SolvedLib An Endothermic Solution Process Is Described By Which Of The Following when a solvent is added to a solution, steps 1 and 2 are both endothermic because energy is required to overcome the intermolecular interactions in the solvent ( δh1) and the solute ( δh2 ). in the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. Click. An Endothermic Solution Process Is Described By Which Of The Following.
From chhattisgarh.pscnotes.com
Exothermic and endothermic reactions CGPCS Exam Preparation An Endothermic Solution Process Is Described By Which Of The Following Heat energy is absorbed from the pan to cook the egg. Click the card to flip 👆. an endothermic solution process is described by which of the following? B) δh > 0, solution feels hot. Click the card to flip 👆. The opposite of an endothermic. in the course of an endothermic process, the system gains heat from. An Endothermic Solution Process Is Described By Which Of The Following.