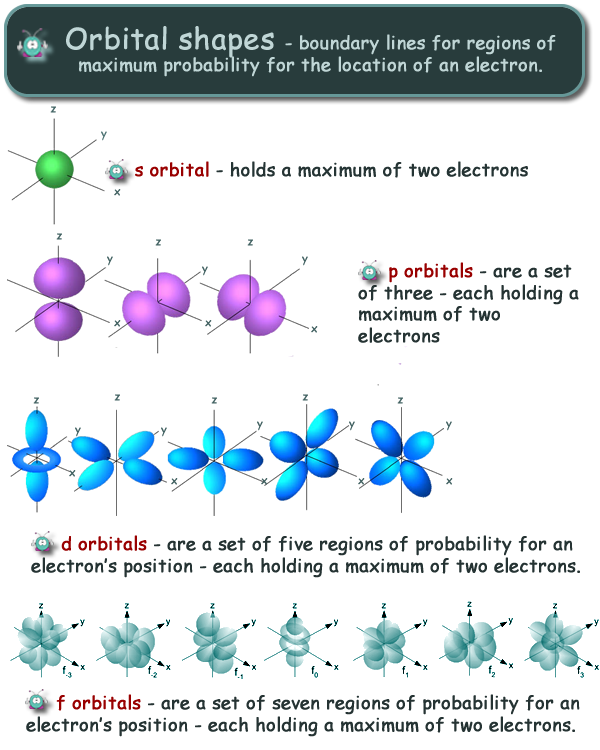
Difference Between Orbital And Shell . Each shell is subdivided into subshells, which are made up of orbitals, each of. In order to move between shells, an electron must absorb or release an. The principal quantum number (n), the. what are shells, subshells, and orbitals? an orbital is a space where a specific pair of electrons can be. Each orbital is characterized by three quantum numbers: by convention, each shell is assigned a number and the symbol n—for example, the electron shell closest to the nucleus is called 1n. shells, subshells, and orbitals | atomic structure and properties | ap. an orbital is a region in space around the nucleus of an atom where there is a high probability of finding an electron.
from www.cyberphysics.co.uk
an orbital is a region in space around the nucleus of an atom where there is a high probability of finding an electron. an orbital is a space where a specific pair of electrons can be. shells, subshells, and orbitals | atomic structure and properties | ap. Each shell is subdivided into subshells, which are made up of orbitals, each of. what are shells, subshells, and orbitals? by convention, each shell is assigned a number and the symbol n—for example, the electron shell closest to the nucleus is called 1n. In order to move between shells, an electron must absorb or release an. Each orbital is characterized by three quantum numbers: The principal quantum number (n), the.
Physics revision GCSE and A Level Physics Revision Cyberphysics
Difference Between Orbital And Shell In order to move between shells, an electron must absorb or release an. The principal quantum number (n), the. what are shells, subshells, and orbitals? Each shell is subdivided into subshells, which are made up of orbitals, each of. an orbital is a region in space around the nucleus of an atom where there is a high probability of finding an electron. Each orbital is characterized by three quantum numbers: an orbital is a space where a specific pair of electrons can be. In order to move between shells, an electron must absorb or release an. shells, subshells, and orbitals | atomic structure and properties | ap. by convention, each shell is assigned a number and the symbol n—for example, the electron shell closest to the nucleus is called 1n.
From www.chemistrystudent.com
Electron Orbitals (ALevel) ChemistryStudent Difference Between Orbital And Shell Each orbital is characterized by three quantum numbers: what are shells, subshells, and orbitals? In order to move between shells, an electron must absorb or release an. shells, subshells, and orbitals | atomic structure and properties | ap. an orbital is a space where a specific pair of electrons can be. by convention, each shell is. Difference Between Orbital And Shell.
From www.youtube.com
ORBIT vs ORBITAL electrons 3min Quick short differences YouTube Difference Between Orbital And Shell by convention, each shell is assigned a number and the symbol n—for example, the electron shell closest to the nucleus is called 1n. Each orbital is characterized by three quantum numbers: Each shell is subdivided into subshells, which are made up of orbitals, each of. shells, subshells, and orbitals | atomic structure and properties | ap. The principal. Difference Between Orbital And Shell.
From polizhuge.weebly.com
Atomic orbitals explained polizhuge Difference Between Orbital And Shell In order to move between shells, an electron must absorb or release an. shells, subshells, and orbitals | atomic structure and properties | ap. Each shell is subdivided into subshells, which are made up of orbitals, each of. Each orbital is characterized by three quantum numbers: an orbital is a space where a specific pair of electrons can. Difference Between Orbital And Shell.
From www.youtube.com
Shells, Subshells, and Orbitals l Understand the difference YouTube Difference Between Orbital And Shell an orbital is a space where a specific pair of electrons can be. In order to move between shells, an electron must absorb or release an. by convention, each shell is assigned a number and the symbol n—for example, the electron shell closest to the nucleus is called 1n. shells, subshells, and orbitals | atomic structure and. Difference Between Orbital And Shell.
From chemistrymsq11.blogspot.com
Grade 11 CHAPTER 1 ATOMIC STRUCTURE SEMESTER1 Difference Between Orbital And Shell Each shell is subdivided into subshells, which are made up of orbitals, each of. an orbital is a region in space around the nucleus of an atom where there is a high probability of finding an electron. shells, subshells, and orbitals | atomic structure and properties | ap. an orbital is a space where a specific pair. Difference Between Orbital And Shell.
From newtondesk.com
Periodic Elements Electron Shells, SubShells, and Orbitals Chemistry Difference Between Orbital And Shell by convention, each shell is assigned a number and the symbol n—for example, the electron shell closest to the nucleus is called 1n. In order to move between shells, an electron must absorb or release an. The principal quantum number (n), the. an orbital is a region in space around the nucleus of an atom where there is. Difference Between Orbital And Shell.
From socratic.org
What is the difference between Shell (orbit) , Subshell and orbital Difference Between Orbital And Shell The principal quantum number (n), the. Each shell is subdivided into subshells, which are made up of orbitals, each of. an orbital is a region in space around the nucleus of an atom where there is a high probability of finding an electron. what are shells, subshells, and orbitals? an orbital is a space where a specific. Difference Between Orbital And Shell.
From www.coscinecreative.com
What is the Difference in a Shell, Subshell and Orbital? — CoScine Creative Difference Between Orbital And Shell Each orbital is characterized by three quantum numbers: In order to move between shells, an electron must absorb or release an. by convention, each shell is assigned a number and the symbol n—for example, the electron shell closest to the nucleus is called 1n. The principal quantum number (n), the. Each shell is subdivided into subshells, which are made. Difference Between Orbital And Shell.
From www.teachoo.com
Distribution of Electrons in Different Orbits [with Examples] Teacho Difference Between Orbital And Shell In order to move between shells, an electron must absorb or release an. by convention, each shell is assigned a number and the symbol n—for example, the electron shell closest to the nucleus is called 1n. shells, subshells, and orbitals | atomic structure and properties | ap. an orbital is a space where a specific pair of. Difference Between Orbital And Shell.
From www.slideserve.com
PPT Chapter Three PowerPoint Presentation, free download ID5840496 Difference Between Orbital And Shell The principal quantum number (n), the. Each shell is subdivided into subshells, which are made up of orbitals, each of. Each orbital is characterized by three quantum numbers: an orbital is a region in space around the nucleus of an atom where there is a high probability of finding an electron. what are shells, subshells, and orbitals? In. Difference Between Orbital And Shell.
From newtondesk.com
Periodic Elements Electron Shells, SubShells, and Orbitals Chemistry Difference Between Orbital And Shell what are shells, subshells, and orbitals? an orbital is a region in space around the nucleus of an atom where there is a high probability of finding an electron. by convention, each shell is assigned a number and the symbol n—for example, the electron shell closest to the nucleus is called 1n. an orbital is a. Difference Between Orbital And Shell.
From www.youtube.com
Structure of Atom Class 11 Chemistry Difference between orbit and Difference Between Orbital And Shell Each orbital is characterized by three quantum numbers: In order to move between shells, an electron must absorb or release an. by convention, each shell is assigned a number and the symbol n—for example, the electron shell closest to the nucleus is called 1n. shells, subshells, and orbitals | atomic structure and properties | ap. The principal quantum. Difference Between Orbital And Shell.
From pressbooks.bccampus.ca
8.3 Development of Quantum Theory CHEM 1114 Introduction to Chemistry Difference Between Orbital And Shell The principal quantum number (n), the. Each shell is subdivided into subshells, which are made up of orbitals, each of. Each orbital is characterized by three quantum numbers: In order to move between shells, an electron must absorb or release an. an orbital is a region in space around the nucleus of an atom where there is a high. Difference Between Orbital And Shell.
From chem.libretexts.org
2.2 Electron Configurations Chemistry LibreTexts Difference Between Orbital And Shell what are shells, subshells, and orbitals? The principal quantum number (n), the. an orbital is a space where a specific pair of electrons can be. an orbital is a region in space around the nucleus of an atom where there is a high probability of finding an electron. shells, subshells, and orbitals | atomic structure and. Difference Between Orbital And Shell.
From slideplayer.com
Shells and Subshells The orbitals in an atom are arranged in shells and Difference Between Orbital And Shell Each shell is subdivided into subshells, which are made up of orbitals, each of. shells, subshells, and orbitals | atomic structure and properties | ap. an orbital is a space where a specific pair of electrons can be. an orbital is a region in space around the nucleus of an atom where there is a high probability. Difference Between Orbital And Shell.
From chem.libretexts.org
6.6 3D Representation of Orbitals Chemistry LibreTexts Difference Between Orbital And Shell an orbital is a region in space around the nucleus of an atom where there is a high probability of finding an electron. The principal quantum number (n), the. In order to move between shells, an electron must absorb or release an. by convention, each shell is assigned a number and the symbol n—for example, the electron shell. Difference Between Orbital And Shell.
From www.i-ciencias.com
quantummechanics ¿Qué representan los orbitales atómicos Difference Between Orbital And Shell an orbital is a region in space around the nucleus of an atom where there is a high probability of finding an electron. an orbital is a space where a specific pair of electrons can be. In order to move between shells, an electron must absorb or release an. Each orbital is characterized by three quantum numbers: . Difference Between Orbital And Shell.
From www.cyberphysics.co.uk
Physics revision GCSE and A Level Physics Revision Cyberphysics Difference Between Orbital And Shell an orbital is a space where a specific pair of electrons can be. by convention, each shell is assigned a number and the symbol n—for example, the electron shell closest to the nucleus is called 1n. shells, subshells, and orbitals | atomic structure and properties | ap. an orbital is a region in space around the. Difference Between Orbital And Shell.
From pediaa.com
Difference Between Shell Subshell and Orbital Definition, Structure Difference Between Orbital And Shell shells, subshells, and orbitals | atomic structure and properties | ap. In order to move between shells, an electron must absorb or release an. an orbital is a space where a specific pair of electrons can be. what are shells, subshells, and orbitals? The principal quantum number (n), the. by convention, each shell is assigned a. Difference Between Orbital And Shell.
From brainly.in
differentiate between shell subshell and orbitals with the help of Difference Between Orbital And Shell an orbital is a region in space around the nucleus of an atom where there is a high probability of finding an electron. what are shells, subshells, and orbitals? In order to move between shells, an electron must absorb or release an. by convention, each shell is assigned a number and the symbol n—for example, the electron. Difference Between Orbital And Shell.
From www.youtube.com
What is the Difference between Shell and Subshell Chemistry Class 9 Difference Between Orbital And Shell an orbital is a space where a specific pair of electrons can be. an orbital is a region in space around the nucleus of an atom where there is a high probability of finding an electron. In order to move between shells, an electron must absorb or release an. shells, subshells, and orbitals | atomic structure and. Difference Between Orbital And Shell.
From pediaa.com
Difference Between Orbitals and Energy Levels Formation, Properties Difference Between Orbital And Shell shells, subshells, and orbitals | atomic structure and properties | ap. what are shells, subshells, and orbitals? an orbital is a region in space around the nucleus of an atom where there is a high probability of finding an electron. The principal quantum number (n), the. Each shell is subdivided into subshells, which are made up of. Difference Between Orbital And Shell.
From pijaeducation.com
ATOM, ORBITS AND ENERGY LEVELS » PIJA Education Difference Between Orbital And Shell In order to move between shells, an electron must absorb or release an. what are shells, subshells, and orbitals? an orbital is a space where a specific pair of electrons can be. shells, subshells, and orbitals | atomic structure and properties | ap. Each orbital is characterized by three quantum numbers: an orbital is a region. Difference Between Orbital And Shell.
From guweb2.gonzaga.edu
CHEM 101 Lecture 7 Difference Between Orbital And Shell Each orbital is characterized by three quantum numbers: by convention, each shell is assigned a number and the symbol n—for example, the electron shell closest to the nucleus is called 1n. what are shells, subshells, and orbitals? an orbital is a region in space around the nucleus of an atom where there is a high probability of. Difference Between Orbital And Shell.
From www.youtube.com
Orbits, Shells, Subshells and Orbitals CSIR Life Sciences CYTOLOGY Difference Between Orbital And Shell Each shell is subdivided into subshells, which are made up of orbitals, each of. shells, subshells, and orbitals | atomic structure and properties | ap. what are shells, subshells, and orbitals? an orbital is a space where a specific pair of electrons can be. Each orbital is characterized by three quantum numbers: In order to move between. Difference Between Orbital And Shell.
From www.slideserve.com
PPT 1. Structure and Bonding PowerPoint Presentation, free download Difference Between Orbital And Shell shells, subshells, and orbitals | atomic structure and properties | ap. Each shell is subdivided into subshells, which are made up of orbitals, each of. an orbital is a space where a specific pair of electrons can be. In order to move between shells, an electron must absorb or release an. what are shells, subshells, and orbitals?. Difference Between Orbital And Shell.
From www.youtube.com
Shells, Subshells and Orbitals YouTube Difference Between Orbital And Shell an orbital is a region in space around the nucleus of an atom where there is a high probability of finding an electron. The principal quantum number (n), the. what are shells, subshells, and orbitals? shells, subshells, and orbitals | atomic structure and properties | ap. Each shell is subdivided into subshells, which are made up of. Difference Between Orbital And Shell.
From brainly.in
differentiate between shell subshell and orbitals with the help of Difference Between Orbital And Shell In order to move between shells, an electron must absorb or release an. by convention, each shell is assigned a number and the symbol n—for example, the electron shell closest to the nucleus is called 1n. an orbital is a space where a specific pair of electrons can be. what are shells, subshells, and orbitals? The principal. Difference Between Orbital And Shell.
From www.youtube.com
What is Difference between Shell, Sub shell and Orbital Structure Difference Between Orbital And Shell what are shells, subshells, and orbitals? an orbital is a region in space around the nucleus of an atom where there is a high probability of finding an electron. shells, subshells, and orbitals | atomic structure and properties | ap. by convention, each shell is assigned a number and the symbol n—for example, the electron shell. Difference Between Orbital And Shell.
From chemaddicts.blogspot.com
Chemaddicts Atoms, shells ,Subshells and Orbitals Difference Between Orbital And Shell an orbital is a space where a specific pair of electrons can be. by convention, each shell is assigned a number and the symbol n—for example, the electron shell closest to the nucleus is called 1n. The principal quantum number (n), the. shells, subshells, and orbitals | atomic structure and properties | ap. Each orbital is characterized. Difference Between Orbital And Shell.
From byjus.com
what is the sequence of shell , subshell, orbitals , orbits , electrons Difference Between Orbital And Shell In order to move between shells, an electron must absorb or release an. by convention, each shell is assigned a number and the symbol n—for example, the electron shell closest to the nucleus is called 1n. an orbital is a region in space around the nucleus of an atom where there is a high probability of finding an. Difference Between Orbital And Shell.
From chemistry-nia.blogspot.com
Made Easy Chemistry Difference between shells, subshells and orbitals Difference Between Orbital And Shell an orbital is a space where a specific pair of electrons can be. by convention, each shell is assigned a number and the symbol n—for example, the electron shell closest to the nucleus is called 1n. In order to move between shells, an electron must absorb or release an. what are shells, subshells, and orbitals? an. Difference Between Orbital And Shell.
From taniya-jolpblogmckinney.blogspot.com
How Many Orbitals Are in a P Subshell Difference Between Orbital And Shell The principal quantum number (n), the. Each orbital is characterized by three quantum numbers: an orbital is a region in space around the nucleus of an atom where there is a high probability of finding an electron. an orbital is a space where a specific pair of electrons can be. by convention, each shell is assigned a. Difference Between Orbital And Shell.
From chemistryskills.com
Shapes of Orbitals and their Types Chemistry Skills Difference Between Orbital And Shell Each orbital is characterized by three quantum numbers: by convention, each shell is assigned a number and the symbol n—for example, the electron shell closest to the nucleus is called 1n. shells, subshells, and orbitals | atomic structure and properties | ap. Each shell is subdivided into subshells, which are made up of orbitals, each of. an. Difference Between Orbital And Shell.
From www.youtube.com
Shells, Subshells, and Orbitals, Oh My! YouTube Difference Between Orbital And Shell Each shell is subdivided into subshells, which are made up of orbitals, each of. an orbital is a space where a specific pair of electrons can be. In order to move between shells, an electron must absorb or release an. Each orbital is characterized by three quantum numbers: an orbital is a region in space around the nucleus. Difference Between Orbital And Shell.