Endothermic Reaction Temperature Increase . When temperature is the stress that affects a system at equilibrium, there are two important consequences: A temperature change occurs when temperature is increased or decreased by the flow of heat. This shifts chemical equilibria toward. When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. Thus an increase in temperature would mean an increase in total entropy change, indicating a more favorable change. The rate of the endothermic reaction is increased more than the rate of the backward reaction in response to a change in. In the initial reaction, the energy given off is negative and thus the reaction is exothermic. When energy is absorbed in an endothermic reaction, the temperature. In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system.
from www.numerade.com
When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. This shifts chemical equilibria toward. When temperature is the stress that affects a system at equilibrium, there are two important consequences: The rate of the endothermic reaction is increased more than the rate of the backward reaction in response to a change in. In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. Thus an increase in temperature would mean an increase in total entropy change, indicating a more favorable change. In the initial reaction, the energy given off is negative and thus the reaction is exothermic. When energy is absorbed in an endothermic reaction, the temperature. A temperature change occurs when temperature is increased or decreased by the flow of heat.
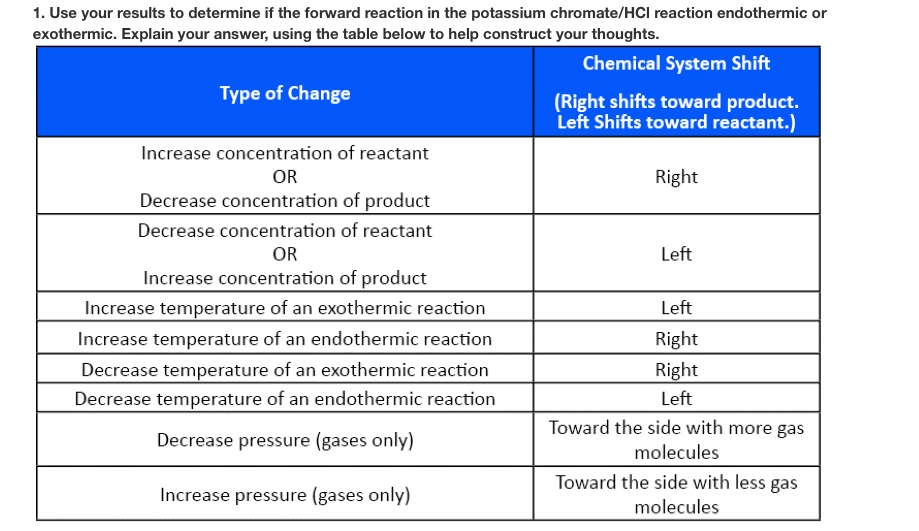
Use your results to determine if the forward reaction in the potassium
Endothermic Reaction Temperature Increase In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. A temperature change occurs when temperature is increased or decreased by the flow of heat. The rate of the endothermic reaction is increased more than the rate of the backward reaction in response to a change in. When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. When energy is absorbed in an endothermic reaction, the temperature. In the initial reaction, the energy given off is negative and thus the reaction is exothermic. This shifts chemical equilibria toward. When temperature is the stress that affects a system at equilibrium, there are two important consequences: Thus an increase in temperature would mean an increase in total entropy change, indicating a more favorable change. In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system.
From revisechemistry.uk
Reversible Reactions and Dynamic Equilibrium AQA C6 revisechemistry.uk Endothermic Reaction Temperature Increase In the initial reaction, the energy given off is negative and thus the reaction is exothermic. When energy is absorbed in an endothermic reaction, the temperature. Thus an increase in temperature would mean an increase in total entropy change, indicating a more favorable change. When temperature is the stress that affects a system at equilibrium, there are two important consequences:. Endothermic Reaction Temperature Increase.
From slidetodoc.com
Chemical Equilibrium Chapter 14 Chemical Equilibrium 14 1 Endothermic Reaction Temperature Increase When energy is absorbed in an endothermic reaction, the temperature. In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. In the initial reaction, the energy given off is negative and thus the reaction is exothermic. When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. Thus an. Endothermic Reaction Temperature Increase.
From www.numerade.com
Use your results to determine if the forward reaction in the potassium Endothermic Reaction Temperature Increase When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. The rate of the endothermic reaction is increased more than the rate of the backward reaction in response to a change in. When temperature is the stress that affects a system at equilibrium, there are two important consequences: Thus an increase in temperature would mean. Endothermic Reaction Temperature Increase.
From eduinput.com
Endothermic ReactionsCharacteristics, Identification, and Examples Endothermic Reaction Temperature Increase When energy is absorbed in an endothermic reaction, the temperature. When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. A temperature change occurs when temperature is increased or decreased by the flow of heat. This shifts chemical. Endothermic Reaction Temperature Increase.
From slideplayer.com
Endothermic & Exothermic Reactions ppt download Endothermic Reaction Temperature Increase When temperature is the stress that affects a system at equilibrium, there are two important consequences: This shifts chemical equilibria toward. When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. A temperature change occurs when temperature is increased or decreased by the flow of heat. The rate of the endothermic reaction is increased more. Endothermic Reaction Temperature Increase.
From quotecentralage.blogspot.com
The Best 20 Endothermic Reaction Graphs quotecentralage Endothermic Reaction Temperature Increase In the initial reaction, the energy given off is negative and thus the reaction is exothermic. The rate of the endothermic reaction is increased more than the rate of the backward reaction in response to a change in. When energy is absorbed in an endothermic reaction, the temperature. Thus an increase in temperature would mean an increase in total entropy. Endothermic Reaction Temperature Increase.
From www.slideserve.com
PPT Section 8.4—Le Chatelier’s Principle PowerPoint Presentation ID Endothermic Reaction Temperature Increase In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. The rate of the endothermic reaction is increased more than the rate of the backward reaction in response to a change in. When energy is absorbed in an endothermic reaction, the temperature. This shifts chemical equilibria toward. A temperature change occurs when temperature. Endothermic Reaction Temperature Increase.
From sciencenotes.org
Endothermic Reactions Definition and Examples Endothermic Reaction Temperature Increase This shifts chemical equilibria toward. When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. When temperature is the stress that affects a system at equilibrium, there are two important consequences: When energy is absorbed in an endothermic reaction, the temperature. In the initial reaction, the energy given off is negative and thus the reaction. Endothermic Reaction Temperature Increase.
From facts.net
16 Intriguing Facts About Endothermic Endothermic Reaction Temperature Increase When energy is absorbed in an endothermic reaction, the temperature. The rate of the endothermic reaction is increased more than the rate of the backward reaction in response to a change in. Thus an increase in temperature would mean an increase in total entropy change, indicating a more favorable change. In the initial reaction, the energy given off is negative. Endothermic Reaction Temperature Increase.
From slideplayer.com
Endothermic Vs. Exothermic Reaction Graphs ppt download Endothermic Reaction Temperature Increase When temperature is the stress that affects a system at equilibrium, there are two important consequences: In the initial reaction, the energy given off is negative and thus the reaction is exothermic. A temperature change occurs when temperature is increased or decreased by the flow of heat. Thus an increase in temperature would mean an increase in total entropy change,. Endothermic Reaction Temperature Increase.
From slideplayer.com
REACTION E. Schnobrich. ppt download Endothermic Reaction Temperature Increase In the initial reaction, the energy given off is negative and thus the reaction is exothermic. Thus an increase in temperature would mean an increase in total entropy change, indicating a more favorable change. A temperature change occurs when temperature is increased or decreased by the flow of heat. When temperature is the stress that affects a system at equilibrium,. Endothermic Reaction Temperature Increase.
From www.bartleby.com
Answered For an endothermic reaction in… bartleby Endothermic Reaction Temperature Increase This shifts chemical equilibria toward. Thus an increase in temperature would mean an increase in total entropy change, indicating a more favorable change. When energy is absorbed in an endothermic reaction, the temperature. A temperature change occurs when temperature is increased or decreased by the flow of heat. When temperature is the stress that affects a system at equilibrium, there. Endothermic Reaction Temperature Increase.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction Temperature Increase Thus an increase in temperature would mean an increase in total entropy change, indicating a more favorable change. When temperature is the stress that affects a system at equilibrium, there are two important consequences: This shifts chemical equilibria toward. When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. In the initial reaction, the energy. Endothermic Reaction Temperature Increase.
From ar.inspiredpencil.com
Endothermic And Exothermic Reactions Temperature Change Endothermic Reaction Temperature Increase When temperature is the stress that affects a system at equilibrium, there are two important consequences: When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. Thus an increase in temperature would mean an increase in total entropy change, indicating a more favorable change. In an endothermic process, the heat that a system absorbs is. Endothermic Reaction Temperature Increase.
From slideplayer.com
Reaction Rates and Le Chatelier’s Principle ppt download Endothermic Reaction Temperature Increase In the initial reaction, the energy given off is negative and thus the reaction is exothermic. When temperature is the stress that affects a system at equilibrium, there are two important consequences: The rate of the endothermic reaction is increased more than the rate of the backward reaction in response to a change in. This shifts chemical equilibria toward. When. Endothermic Reaction Temperature Increase.
From slideplayer.com
Qualitative Changes in Equilibrium Systems ppt download Endothermic Reaction Temperature Increase When energy is absorbed in an endothermic reaction, the temperature. In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. Thus an increase in temperature would mean an increase in total entropy change, indicating a more favorable change. A temperature change occurs when temperature is increased or decreased by the flow of heat.. Endothermic Reaction Temperature Increase.
From www.slideserve.com
PPT Exothermic and Endothermic Reactions PowerPoint Presentation Endothermic Reaction Temperature Increase When temperature is the stress that affects a system at equilibrium, there are two important consequences: In the initial reaction, the energy given off is negative and thus the reaction is exothermic. A temperature change occurs when temperature is increased or decreased by the flow of heat. When energy is released in an exothermic reaction, the temperature of the reaction. Endothermic Reaction Temperature Increase.
From muadacsan3mien.com
What Are Exothermic And Endothermic Changes? Examples Unveiled! Endothermic Reaction Temperature Increase In the initial reaction, the energy given off is negative and thus the reaction is exothermic. This shifts chemical equilibria toward. When energy is absorbed in an endothermic reaction, the temperature. When temperature is the stress that affects a system at equilibrium, there are two important consequences: A temperature change occurs when temperature is increased or decreased by the flow. Endothermic Reaction Temperature Increase.
From ar.inspiredpencil.com
Endothermic And Exothermic Reactions Temperature Change Endothermic Reaction Temperature Increase When energy is absorbed in an endothermic reaction, the temperature. When temperature is the stress that affects a system at equilibrium, there are two important consequences: In the initial reaction, the energy given off is negative and thus the reaction is exothermic. This shifts chemical equilibria toward. A temperature change occurs when temperature is increased or decreased by the flow. Endothermic Reaction Temperature Increase.
From www.vrogue.co
Endothermic And Exothermic Reactions Multiple Choice vrogue.co Endothermic Reaction Temperature Increase When energy is absorbed in an endothermic reaction, the temperature. In the initial reaction, the energy given off is negative and thus the reaction is exothermic. This shifts chemical equilibria toward. In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. When energy is released in an exothermic reaction, the temperature of the. Endothermic Reaction Temperature Increase.
From byjus.com
Difference Between Endothermic and Exothermic Reactions Chemistry Endothermic Reaction Temperature Increase Thus an increase in temperature would mean an increase in total entropy change, indicating a more favorable change. The rate of the endothermic reaction is increased more than the rate of the backward reaction in response to a change in. A temperature change occurs when temperature is increased or decreased by the flow of heat. When energy is absorbed in. Endothermic Reaction Temperature Increase.
From www.youtube.com
Le Chatelier's Principle and Temperature Changes (Pt. 10) YouTube Endothermic Reaction Temperature Increase In the initial reaction, the energy given off is negative and thus the reaction is exothermic. A temperature change occurs when temperature is increased or decreased by the flow of heat. In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. When temperature is the stress that affects a system at equilibrium, there. Endothermic Reaction Temperature Increase.
From www.expii.com
Endothermic and Exothermic Reactions — Overview & Comparison Expii Endothermic Reaction Temperature Increase A temperature change occurs when temperature is increased or decreased by the flow of heat. When temperature is the stress that affects a system at equilibrium, there are two important consequences: Thus an increase in temperature would mean an increase in total entropy change, indicating a more favorable change. The rate of the endothermic reaction is increased more than the. Endothermic Reaction Temperature Increase.
From byjus.com
45. Explain why rate of exothermic reaction increases with increasing Endothermic Reaction Temperature Increase When temperature is the stress that affects a system at equilibrium, there are two important consequences: When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. In the initial reaction, the energy given off is negative and thus the reaction is exothermic. The rate of the endothermic reaction is increased more than the rate of. Endothermic Reaction Temperature Increase.
From en.ppt-online.org
Thermal Energy, Chemical Energy online presentation Endothermic Reaction Temperature Increase Thus an increase in temperature would mean an increase in total entropy change, indicating a more favorable change. In the initial reaction, the energy given off is negative and thus the reaction is exothermic. When energy is absorbed in an endothermic reaction, the temperature. When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. A. Endothermic Reaction Temperature Increase.
From www.tes.com
Endothermic and Exothermic Temperature Changes Edexcel 91 Teaching Endothermic Reaction Temperature Increase The rate of the endothermic reaction is increased more than the rate of the backward reaction in response to a change in. In the initial reaction, the energy given off is negative and thus the reaction is exothermic. When energy is absorbed in an endothermic reaction, the temperature. When energy is released in an exothermic reaction, the temperature of the. Endothermic Reaction Temperature Increase.
From ar.inspiredpencil.com
Endothermic And Exothermic Reactions Temperature Change Endothermic Reaction Temperature Increase Thus an increase in temperature would mean an increase in total entropy change, indicating a more favorable change. A temperature change occurs when temperature is increased or decreased by the flow of heat. The rate of the endothermic reaction is increased more than the rate of the backward reaction in response to a change in. When energy is absorbed in. Endothermic Reaction Temperature Increase.
From revisechemistry.uk
Exothermic and Endothermic Reactions AQA C5 revisechemistry.uk Endothermic Reaction Temperature Increase The rate of the endothermic reaction is increased more than the rate of the backward reaction in response to a change in. In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. In the initial reaction, the energy given off is negative and thus the reaction is exothermic. When temperature is the stress. Endothermic Reaction Temperature Increase.
From www.w3schools.blog
Factors affecting the rate of a reaction Temperature W3schools Endothermic Reaction Temperature Increase Thus an increase in temperature would mean an increase in total entropy change, indicating a more favorable change. In the initial reaction, the energy given off is negative and thus the reaction is exothermic. When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. The rate of the endothermic reaction is increased more than the. Endothermic Reaction Temperature Increase.
From www.slideserve.com
PPT Le Chatelier’s Principle PowerPoint Presentation, free download Endothermic Reaction Temperature Increase The rate of the endothermic reaction is increased more than the rate of the backward reaction in response to a change in. When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. When temperature is the stress that affects a system at equilibrium, there are two important consequences: When energy is absorbed in an endothermic. Endothermic Reaction Temperature Increase.
From www.vrogue.co
Identify The Endothermic And Exothermic Reaction vrogue.co Endothermic Reaction Temperature Increase In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. In the initial reaction, the energy given off is negative and thus the reaction is exothermic. This shifts chemical equilibria toward. Thus an increase in temperature would mean an increase in total entropy change, indicating a more favorable change. The rate of the. Endothermic Reaction Temperature Increase.
From www.baamboozle.com
Energy Change and Reaction Rate Baamboozle Baamboozle The Most Endothermic Reaction Temperature Increase This shifts chemical equilibria toward. When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. When temperature is the stress that affects a system at equilibrium, there are two important consequences: In the initial reaction, the energy given off is negative and thus the reaction is exothermic. The rate of the endothermic reaction is increased. Endothermic Reaction Temperature Increase.
From www.worksheetsplanet.com
What is an Endothermic Reaction Definition & Example Endothermic Reaction Temperature Increase A temperature change occurs when temperature is increased or decreased by the flow of heat. When energy is absorbed in an endothermic reaction, the temperature. The rate of the endothermic reaction is increased more than the rate of the backward reaction in response to a change in. This shifts chemical equilibria toward. When energy is released in an exothermic reaction,. Endothermic Reaction Temperature Increase.
From slideplayer.com
Qualitative Changes in Equilibrium Systems ppt download Endothermic Reaction Temperature Increase In the initial reaction, the energy given off is negative and thus the reaction is exothermic. Thus an increase in temperature would mean an increase in total entropy change, indicating a more favorable change. When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. In an endothermic process, the heat that a system absorbs is. Endothermic Reaction Temperature Increase.
From improove.in
Experiment 16 Heat generation during reaction Improove Endothermic Reaction Temperature Increase The rate of the endothermic reaction is increased more than the rate of the backward reaction in response to a change in. When energy is released in an exothermic reaction, the temperature of the reaction mixture increases. When energy is absorbed in an endothermic reaction, the temperature. Thus an increase in temperature would mean an increase in total entropy change,. Endothermic Reaction Temperature Increase.