What Is A Class 4 Medical Device In Canada . Class i presents the lowest potential harm; The medical devices regulations include rules to help classify devices into four risk classes. Performs medical device classification and registration of class ii, class iii and class iv with health canada. All the medical devices are classified into four main. An importer is any person in canada, other than the manufacturer of a medical device, who is responsible for the medical device coming into. In canada, medical devices are classified into four categories—class i, ii, iii, and iv—based on their risk levels, with class i. Manufacturers of a class ii, iii or iv medical device must hold a medical device licence (mdl) to import or distribute their own medical device.
from www.greenlight.guru
The medical devices regulations include rules to help classify devices into four risk classes. All the medical devices are classified into four main. Performs medical device classification and registration of class ii, class iii and class iv with health canada. Class i presents the lowest potential harm; Manufacturers of a class ii, iii or iv medical device must hold a medical device licence (mdl) to import or distribute their own medical device. An importer is any person in canada, other than the manufacturer of a medical device, who is responsible for the medical device coming into. In canada, medical devices are classified into four categories—class i, ii, iii, and iv—based on their risk levels, with class i.
Medical Device Classifications Determine Your Device Class
What Is A Class 4 Medical Device In Canada The medical devices regulations include rules to help classify devices into four risk classes. Class i presents the lowest potential harm; The medical devices regulations include rules to help classify devices into four risk classes. Performs medical device classification and registration of class ii, class iii and class iv with health canada. Manufacturers of a class ii, iii or iv medical device must hold a medical device licence (mdl) to import or distribute their own medical device. An importer is any person in canada, other than the manufacturer of a medical device, who is responsible for the medical device coming into. All the medical devices are classified into four main. In canada, medical devices are classified into four categories—class i, ii, iii, and iv—based on their risk levels, with class i.
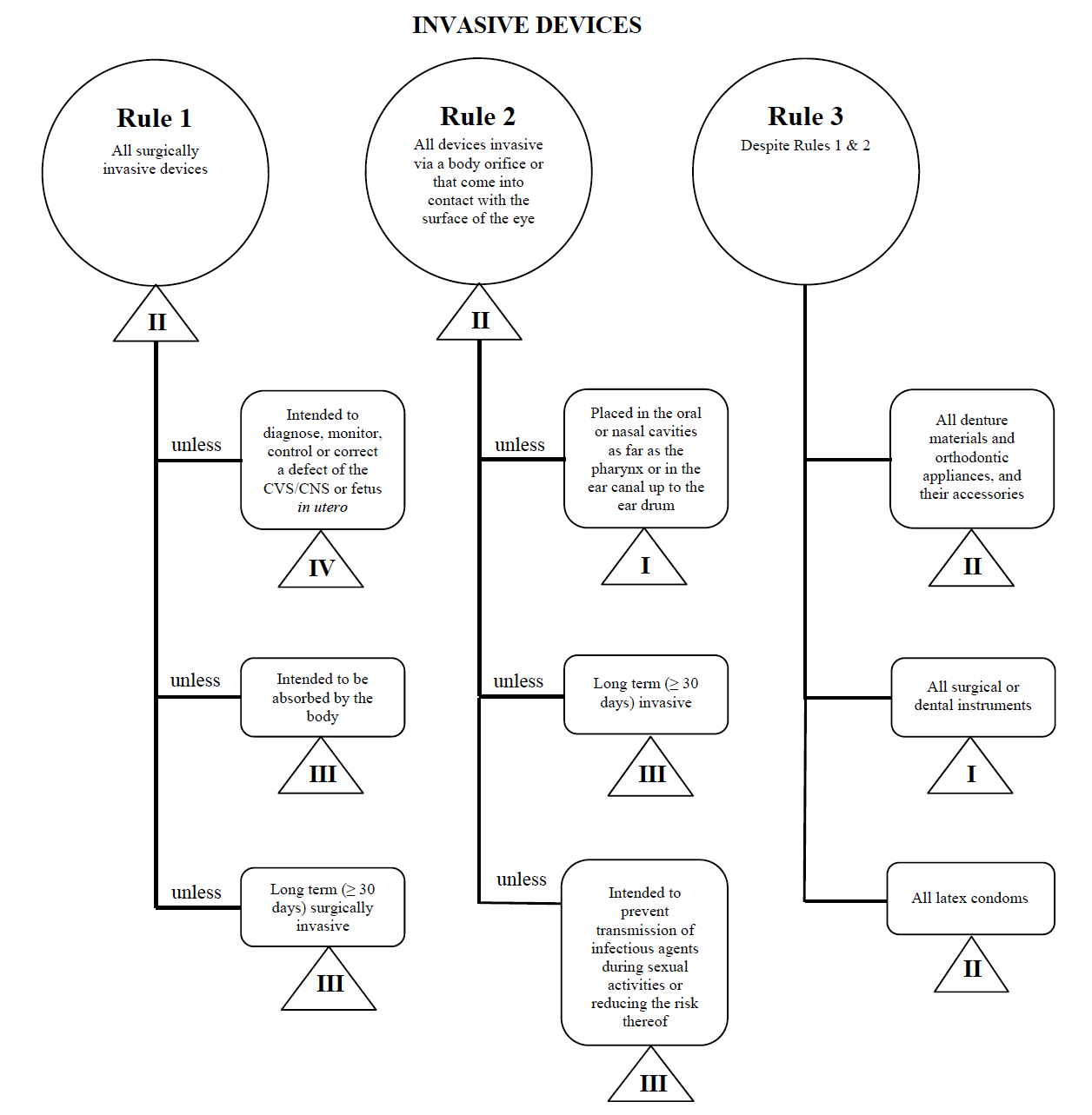
From coastbiomed.com
UNDERSTANDING MEDICAL EQUIPMENT CLASSIFICATION Coast Biomedical Equipment What Is A Class 4 Medical Device In Canada The medical devices regulations include rules to help classify devices into four risk classes. In canada, medical devices are classified into four categories—class i, ii, iii, and iv—based on their risk levels, with class i. All the medical devices are classified into four main. Performs medical device classification and registration of class ii, class iii and class iv with health. What Is A Class 4 Medical Device In Canada.
From www.arenasolutions.com
Class I Device Definition Arena What Is A Class 4 Medical Device In Canada Class i presents the lowest potential harm; All the medical devices are classified into four main. The medical devices regulations include rules to help classify devices into four risk classes. Manufacturers of a class ii, iii or iv medical device must hold a medical device licence (mdl) to import or distribute their own medical device. In canada, medical devices are. What Is A Class 4 Medical Device In Canada.
From omcmedical.com
Classification of Medical Devices Based on UK MDR 2002 What Is A Class 4 Medical Device In Canada Class i presents the lowest potential harm; In canada, medical devices are classified into four categories—class i, ii, iii, and iv—based on their risk levels, with class i. Manufacturers of a class ii, iii or iv medical device must hold a medical device licence (mdl) to import or distribute their own medical device. The medical devices regulations include rules to. What Is A Class 4 Medical Device In Canada.
From medium.com
A Quick Guide to Unique Device Identifiers, and their potential to What Is A Class 4 Medical Device In Canada Manufacturers of a class ii, iii or iv medical device must hold a medical device licence (mdl) to import or distribute their own medical device. All the medical devices are classified into four main. Performs medical device classification and registration of class ii, class iii and class iv with health canada. Class i presents the lowest potential harm; The medical. What Is A Class 4 Medical Device In Canada.
From mavink.com
Mdr Classification Chart What Is A Class 4 Medical Device In Canada The medical devices regulations include rules to help classify devices into four risk classes. An importer is any person in canada, other than the manufacturer of a medical device, who is responsible for the medical device coming into. Class i presents the lowest potential harm; All the medical devices are classified into four main. Performs medical device classification and registration. What Is A Class 4 Medical Device In Canada.
From talema.com
An Introduction to Medical Electrical Devices The Talema Group What Is A Class 4 Medical Device In Canada All the medical devices are classified into four main. An importer is any person in canada, other than the manufacturer of a medical device, who is responsible for the medical device coming into. In canada, medical devices are classified into four categories—class i, ii, iii, and iv—based on their risk levels, with class i. The medical devices regulations include rules. What Is A Class 4 Medical Device In Canada.
From www.pacificbridgemedical.com
Device Classification in India Infographic What Is A Class 4 Medical Device In Canada All the medical devices are classified into four main. Class i presents the lowest potential harm; Manufacturers of a class ii, iii or iv medical device must hold a medical device licence (mdl) to import or distribute their own medical device. An importer is any person in canada, other than the manufacturer of a medical device, who is responsible for. What Is A Class 4 Medical Device In Canada.
From medicaldialogues.in
Indian medical device market expected to touch USD 50 billion by 2025 What Is A Class 4 Medical Device In Canada In canada, medical devices are classified into four categories—class i, ii, iii, and iv—based on their risk levels, with class i. The medical devices regulations include rules to help classify devices into four risk classes. Performs medical device classification and registration of class ii, class iii and class iv with health canada. Class i presents the lowest potential harm; All. What Is A Class 4 Medical Device In Canada.
From es.slideshare.net
Regulation of Medical Devices in US What Is A Class 4 Medical Device In Canada In canada, medical devices are classified into four categories—class i, ii, iii, and iv—based on their risk levels, with class i. Manufacturers of a class ii, iii or iv medical device must hold a medical device licence (mdl) to import or distribute their own medical device. Class i presents the lowest potential harm; Performs medical device classification and registration of. What Is A Class 4 Medical Device In Canada.
From www.orielstat.com
Class 1 Medical Device Requirements Oriel STAT A MATRIX What Is A Class 4 Medical Device In Canada The medical devices regulations include rules to help classify devices into four risk classes. Class i presents the lowest potential harm; In canada, medical devices are classified into four categories—class i, ii, iii, and iv—based on their risk levels, with class i. All the medical devices are classified into four main. Manufacturers of a class ii, iii or iv medical. What Is A Class 4 Medical Device In Canada.
From synectic.net
Medical Device FDA Regulations Infographic Synectic What Is A Class 4 Medical Device In Canada Manufacturers of a class ii, iii or iv medical device must hold a medical device licence (mdl) to import or distribute their own medical device. All the medical devices are classified into four main. An importer is any person in canada, other than the manufacturer of a medical device, who is responsible for the medical device coming into. In canada,. What Is A Class 4 Medical Device In Canada.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU What Is A Class 4 Medical Device In Canada Manufacturers of a class ii, iii or iv medical device must hold a medical device licence (mdl) to import or distribute their own medical device. The medical devices regulations include rules to help classify devices into four risk classes. Class i presents the lowest potential harm; In canada, medical devices are classified into four categories—class i, ii, iii, and iv—based. What Is A Class 4 Medical Device In Canada.
From fyocylyim.blob.core.windows.net
What Is A Class Iv Medical Device at Arron Cooper blog What Is A Class 4 Medical Device In Canada Manufacturers of a class ii, iii or iv medical device must hold a medical device licence (mdl) to import or distribute their own medical device. Performs medical device classification and registration of class ii, class iii and class iv with health canada. In canada, medical devices are classified into four categories—class i, ii, iii, and iv—based on their risk levels,. What Is A Class 4 Medical Device In Canada.
From blog.sierralabs.com
6 Regulatory Pathways to Bring Your Medical Device to Market What Is A Class 4 Medical Device In Canada In canada, medical devices are classified into four categories—class i, ii, iii, and iv—based on their risk levels, with class i. An importer is any person in canada, other than the manufacturer of a medical device, who is responsible for the medical device coming into. Performs medical device classification and registration of class ii, class iii and class iv with. What Is A Class 4 Medical Device In Canada.
From www.vrogue.co
The 3 Fda Medical Device Classes Differences And Exam vrogue.co What Is A Class 4 Medical Device In Canada Class i presents the lowest potential harm; All the medical devices are classified into four main. Manufacturers of a class ii, iii or iv medical device must hold a medical device licence (mdl) to import or distribute their own medical device. The medical devices regulations include rules to help classify devices into four risk classes. An importer is any person. What Is A Class 4 Medical Device In Canada.
From www.sycaimedical.com
Overview on the regulatory path for software medical devices What Is A Class 4 Medical Device In Canada Performs medical device classification and registration of class ii, class iii and class iv with health canada. Manufacturers of a class ii, iii or iv medical device must hold a medical device licence (mdl) to import or distribute their own medical device. In canada, medical devices are classified into four categories—class i, ii, iii, and iv—based on their risk levels,. What Is A Class 4 Medical Device In Canada.
From www.presentationeze.com
FDA medical device classification PresentationEZE What Is A Class 4 Medical Device In Canada All the medical devices are classified into four main. In canada, medical devices are classified into four categories—class i, ii, iii, and iv—based on their risk levels, with class i. Manufacturers of a class ii, iii or iv medical device must hold a medical device licence (mdl) to import or distribute their own medical device. The medical devices regulations include. What Is A Class 4 Medical Device In Canada.
From japanhpn.org
Japan Health Policy NOW 6.4 Medical Devices What Is A Class 4 Medical Device In Canada Manufacturers of a class ii, iii or iv medical device must hold a medical device licence (mdl) to import or distribute their own medical device. Class i presents the lowest potential harm; All the medical devices are classified into four main. The medical devices regulations include rules to help classify devices into four risk classes. In canada, medical devices are. What Is A Class 4 Medical Device In Canada.
From learn.marsdd.com
Medical device submissions Placing a medical device on the market What Is A Class 4 Medical Device In Canada In canada, medical devices are classified into four categories—class i, ii, iii, and iv—based on their risk levels, with class i. Manufacturers of a class ii, iii or iv medical device must hold a medical device licence (mdl) to import or distribute their own medical device. An importer is any person in canada, other than the manufacturer of a medical. What Is A Class 4 Medical Device In Canada.
From www.slideshare.net
Medical Device FDA Regulations and Classifications infographic What Is A Class 4 Medical Device In Canada The medical devices regulations include rules to help classify devices into four risk classes. An importer is any person in canada, other than the manufacturer of a medical device, who is responsible for the medical device coming into. Manufacturers of a class ii, iii or iv medical device must hold a medical device licence (mdl) to import or distribute their. What Is A Class 4 Medical Device In Canada.
From www.greenlight.guru
What is a Class 2 Medical Device in the US? [+Examples] What Is A Class 4 Medical Device In Canada Performs medical device classification and registration of class ii, class iii and class iv with health canada. Manufacturers of a class ii, iii or iv medical device must hold a medical device licence (mdl) to import or distribute their own medical device. In canada, medical devices are classified into four categories—class i, ii, iii, and iv—based on their risk levels,. What Is A Class 4 Medical Device In Canada.
From medicaldevicehq.com
The Perfect Project Process Medical Device Product Development What Is A Class 4 Medical Device In Canada Performs medical device classification and registration of class ii, class iii and class iv with health canada. Class i presents the lowest potential harm; An importer is any person in canada, other than the manufacturer of a medical device, who is responsible for the medical device coming into. In canada, medical devices are classified into four categories—class i, ii, iii,. What Is A Class 4 Medical Device In Canada.
From www.joharidigital.com
Health Canada Approval Process for Medical Devices StepbyStep Guide What Is A Class 4 Medical Device In Canada Manufacturers of a class ii, iii or iv medical device must hold a medical device licence (mdl) to import or distribute their own medical device. An importer is any person in canada, other than the manufacturer of a medical device, who is responsible for the medical device coming into. The medical devices regulations include rules to help classify devices into. What Is A Class 4 Medical Device In Canada.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU What Is A Class 4 Medical Device In Canada In canada, medical devices are classified into four categories—class i, ii, iii, and iv—based on their risk levels, with class i. All the medical devices are classified into four main. Manufacturers of a class ii, iii or iv medical device must hold a medical device licence (mdl) to import or distribute their own medical device. Performs medical device classification and. What Is A Class 4 Medical Device In Canada.
From www.canada.ca
Guidance on Medical Device Establishment Licensing (GUI0016) Canada.ca What Is A Class 4 Medical Device In Canada An importer is any person in canada, other than the manufacturer of a medical device, who is responsible for the medical device coming into. In canada, medical devices are classified into four categories—class i, ii, iii, and iv—based on their risk levels, with class i. All the medical devices are classified into four main. Manufacturers of a class ii, iii. What Is A Class 4 Medical Device In Canada.
From www.slideserve.com
PPT Medical Device Standards PowerPoint Presentation, free download What Is A Class 4 Medical Device In Canada An importer is any person in canada, other than the manufacturer of a medical device, who is responsible for the medical device coming into. The medical devices regulations include rules to help classify devices into four risk classes. Class i presents the lowest potential harm; All the medical devices are classified into four main. In canada, medical devices are classified. What Is A Class 4 Medical Device In Canada.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU What Is A Class 4 Medical Device In Canada All the medical devices are classified into four main. The medical devices regulations include rules to help classify devices into four risk classes. Performs medical device classification and registration of class ii, class iii and class iv with health canada. An importer is any person in canada, other than the manufacturer of a medical device, who is responsible for the. What Is A Class 4 Medical Device In Canada.
From www.mdpi.com
Energies Free FullText Energy Harvesting in Implantable and What Is A Class 4 Medical Device In Canada Manufacturers of a class ii, iii or iv medical device must hold a medical device licence (mdl) to import or distribute their own medical device. All the medical devices are classified into four main. Performs medical device classification and registration of class ii, class iii and class iv with health canada. In canada, medical devices are classified into four categories—class. What Is A Class 4 Medical Device In Canada.
From www.greenlight.guru
Medical Device Classifications Determine Your Device Class What Is A Class 4 Medical Device In Canada Manufacturers of a class ii, iii or iv medical device must hold a medical device licence (mdl) to import or distribute their own medical device. Class i presents the lowest potential harm; Performs medical device classification and registration of class ii, class iii and class iv with health canada. The medical devices regulations include rules to help classify devices into. What Is A Class 4 Medical Device In Canada.
From laegemiddelstyrelsen.dk
Medical devices What Is A Class 4 Medical Device In Canada Class i presents the lowest potential harm; In canada, medical devices are classified into four categories—class i, ii, iii, and iv—based on their risk levels, with class i. Performs medical device classification and registration of class ii, class iii and class iv with health canada. All the medical devices are classified into four main. The medical devices regulations include rules. What Is A Class 4 Medical Device In Canada.
From news.iscas.co
Interoperability standards for medical device integration in the OR and What Is A Class 4 Medical Device In Canada Class i presents the lowest potential harm; An importer is any person in canada, other than the manufacturer of a medical device, who is responsible for the medical device coming into. Manufacturers of a class ii, iii or iv medical device must hold a medical device licence (mdl) to import or distribute their own medical device. Performs medical device classification. What Is A Class 4 Medical Device In Canada.
From www.vrogue.co
Fda Medical Device Classification vrogue.co What Is A Class 4 Medical Device In Canada All the medical devices are classified into four main. In canada, medical devices are classified into four categories—class i, ii, iii, and iv—based on their risk levels, with class i. Class i presents the lowest potential harm; The medical devices regulations include rules to help classify devices into four risk classes. Performs medical device classification and registration of class ii,. What Is A Class 4 Medical Device In Canada.
From angelanjohnson.com
Medical Devices Angela N Johnson What Is A Class 4 Medical Device In Canada An importer is any person in canada, other than the manufacturer of a medical device, who is responsible for the medical device coming into. All the medical devices are classified into four main. The medical devices regulations include rules to help classify devices into four risk classes. Manufacturers of a class ii, iii or iv medical device must hold a. What Is A Class 4 Medical Device In Canada.
From fyocylyim.blob.core.windows.net
What Is A Class Iv Medical Device at Arron Cooper blog What Is A Class 4 Medical Device In Canada All the medical devices are classified into four main. Performs medical device classification and registration of class ii, class iii and class iv with health canada. An importer is any person in canada, other than the manufacturer of a medical device, who is responsible for the medical device coming into. Class i presents the lowest potential harm; In canada, medical. What Is A Class 4 Medical Device In Canada.
From www.greenlight.guru
What is an FDA Class 1 Medical Device? [+Examples] What Is A Class 4 Medical Device In Canada The medical devices regulations include rules to help classify devices into four risk classes. In canada, medical devices are classified into four categories—class i, ii, iii, and iv—based on their risk levels, with class i. Class i presents the lowest potential harm; Performs medical device classification and registration of class ii, class iii and class iv with health canada. Manufacturers. What Is A Class 4 Medical Device In Canada.