Calorimeter Heat Capacity In Chemistry . For example, when an exothermic. Find out the definitions and. The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the chemical reaction. Calorimeters play a crucial role. The temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced by the. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Apply the first law of thermodynamics to calorimetry. Learn how to calculate and interpret heat and related properties using typical calorimetry data. Importance of calorimeters in chemistry. On the left (a), labeled ‘system exothermic process’, a calorimeter contains a substance that, during an exothermic reaction, releases heat.
from www.tec-science.com
For example, when an exothermic. Calorimeters play a crucial role. On the left (a), labeled ‘system exothermic process’, a calorimeter contains a substance that, during an exothermic reaction, releases heat. Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. Apply the first law of thermodynamics to calorimetry. The temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced by the. Find out the definitions and. Importance of calorimeters in chemistry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.
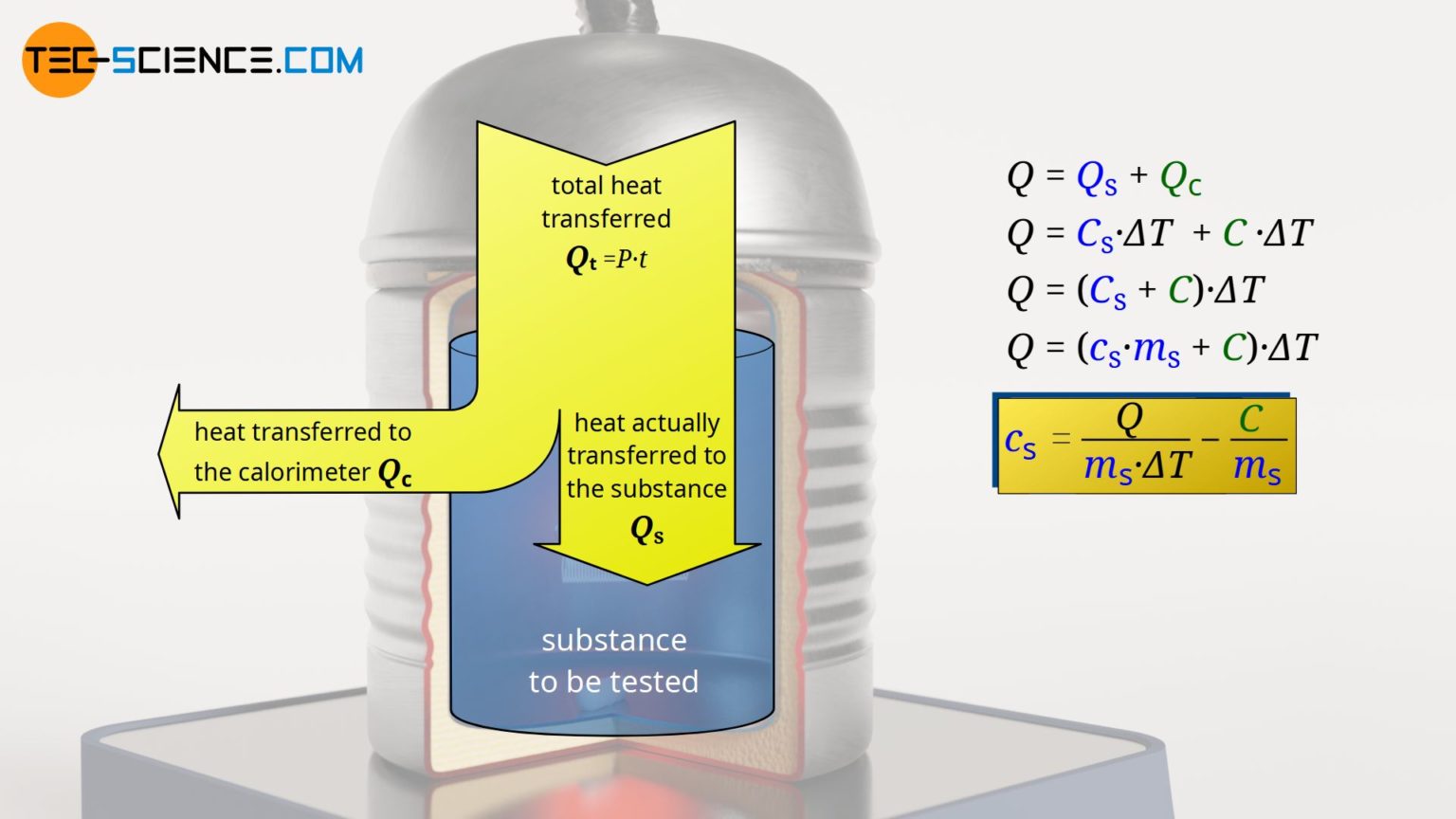
Calorimeter to determine the specific heat capacities of liquids tec
Calorimeter Heat Capacity In Chemistry Find out the definitions and. Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. Importance of calorimeters in chemistry. Calorimeters play a crucial role. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced by the. Find out the definitions and. On the left (a), labeled ‘system exothermic process’, a calorimeter contains a substance that, during an exothermic reaction, releases heat. Apply the first law of thermodynamics to calorimetry. Learn how to calculate and interpret heat and related properties using typical calorimetry data. The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the chemical reaction.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimeter Heat Capacity In Chemistry Importance of calorimeters in chemistry. The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the chemical reaction. The temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced by the. Compare heat flow from. Calorimeter Heat Capacity In Chemistry.
From users.highland.edu
Calorimetry Calorimeter Heat Capacity In Chemistry Find out the definitions and. Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. The temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced by the. Apply the first law of thermodynamics to calorimetry. A calorimeter is a device used to measure. Calorimeter Heat Capacity In Chemistry.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimeter Heat Capacity In Chemistry The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the chemical reaction. Find out the definitions and. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Learn how to calculate and interpret heat and related. Calorimeter Heat Capacity In Chemistry.
From www.savemyexams.com
Calorimetry Experiments SL IB Chemistry Revision Notes 2025 Save My Calorimeter Heat Capacity In Chemistry Learn how to calculate and interpret heat and related properties using typical calorimetry data. For example, when an exothermic. Apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Find out the definitions and. A calorimeter is a device used to measure the amount of heat involved. Calorimeter Heat Capacity In Chemistry.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation Calorimeter Heat Capacity In Chemistry Calorimeters play a crucial role. Find out the definitions and. On the left (a), labeled ‘system exothermic process’, a calorimeter contains a substance that, during an exothermic reaction, releases heat. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Learn how to calculate and interpret heat and related properties using typical calorimetry data. The. Calorimeter Heat Capacity In Chemistry.
From www.youtube.com
CHEMISTRY 101 Constant volume calorimetry YouTube Calorimeter Heat Capacity In Chemistry The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the chemical reaction. Find out the definitions and. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The temperature increase is. Calorimeter Heat Capacity In Chemistry.
From www.slideserve.com
PPT Thermochemistry Chemical Energy PowerPoint Presentation, free Calorimeter Heat Capacity In Chemistry The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the chemical reaction. Importance of calorimeters in chemistry. Apply the first law of thermodynamics to calorimetry. For example, when an exothermic. Find out the definitions and. The temperature increase is measured and, along with the known. Calorimeter Heat Capacity In Chemistry.
From www.learner.org
The Energy in Chemical Reactions Thermodynamics and Enthalpy Calorimeter Heat Capacity In Chemistry The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the chemical reaction. Apply the first law of thermodynamics to calorimetry. Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. The temperature increase is measured and, along with the known. Calorimeter Heat Capacity In Chemistry.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimeter Heat Capacity In Chemistry The temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced by the. On the left (a), labeled ‘system exothermic process’, a calorimeter contains a substance that, during an exothermic reaction, releases heat. The heat capacity of the calorimeter or of the reaction mixture may be used to calculate. Calorimeter Heat Capacity In Chemistry.
From www.youtube.com
G10 Chemistry Calorimetry and Specific Heat Capacity Worked Examples Calorimeter Heat Capacity In Chemistry Find out the definitions and. Calorimeters play a crucial role. On the left (a), labeled ‘system exothermic process’, a calorimeter contains a substance that, during an exothermic reaction, releases heat. Apply the first law of thermodynamics to calorimetry. Importance of calorimeters in chemistry. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat. Calorimeter Heat Capacity In Chemistry.
From www.youtube.com
Final Temperature Calorimetry Practice Problems Chemistry YouTube Calorimeter Heat Capacity In Chemistry Learn how to calculate and interpret heat and related properties using typical calorimetry data. Importance of calorimeters in chemistry. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeters play a crucial role. Apply the first law of thermodynamics to calorimetry. Calorimeters are used to. Calorimeter Heat Capacity In Chemistry.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Calorimeter Heat Capacity In Chemistry Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the chemical reaction. On the left (a), labeled ‘system exothermic process’, a calorimeter contains a substance that, during an exothermic reaction,. Calorimeter Heat Capacity In Chemistry.
From 2012books.lardbucket.org
Calorimetry Calorimeter Heat Capacity In Chemistry Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Find out the definitions and. For example, when an exothermic. Calorimeters play a crucial role. On the left (a), labeled ‘system exothermic process’, a calorimeter contains a substance that, during an exothermic reaction, releases heat. Importance of calorimeters in chemistry. The heat capacity of the. Calorimeter Heat Capacity In Chemistry.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Calorimeter Heat Capacity In Chemistry Find out the definitions and. Importance of calorimeters in chemistry. The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the chemical reaction. Learn how to calculate and interpret heat and related properties using typical calorimetry data. On the left (a), labeled ‘system exothermic process’, a. Calorimeter Heat Capacity In Chemistry.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Calorimeter Heat Capacity In Chemistry Apply the first law of thermodynamics to calorimetry. Importance of calorimeters in chemistry. Learn how to calculate and interpret heat and related properties using typical calorimetry data. Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount. Calorimeter Heat Capacity In Chemistry.
From www.slideshare.net
Tang 01 heat capacity and calorimetry Calorimeter Heat Capacity In Chemistry Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. Apply the first law of thermodynamics to calorimetry. The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or. Calorimeter Heat Capacity In Chemistry.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Calorimeter Heat Capacity In Chemistry The temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced by the. The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the chemical reaction. Find out the definitions and. On the left (a),. Calorimeter Heat Capacity In Chemistry.
From quizdbbarefooted.z21.web.core.windows.net
How To Calculate Calorimeter Calorimeter Heat Capacity In Chemistry On the left (a), labeled ‘system exothermic process’, a calorimeter contains a substance that, during an exothermic reaction, releases heat. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. For example, when an exothermic. The temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the. Calorimeter Heat Capacity In Chemistry.
From porter-yersblogvega.blogspot.com
Heat Capacity of Calorimeter Calorimeter Heat Capacity In Chemistry On the left (a), labeled ‘system exothermic process’, a calorimeter contains a substance that, during an exothermic reaction, releases heat. The temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced by the. Learn how to calculate and interpret heat and related properties using typical calorimetry data. Compare heat. Calorimeter Heat Capacity In Chemistry.
From studylib.net
Calorimetry_and_Specific_Heat_Capacity Calorimeter Heat Capacity In Chemistry Calorimeters play a crucial role. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. Importance of calorimeters in chemistry. Find out the definitions and. Apply the first law of thermodynamics to calorimetry. The heat capacity of the calorimeter or. Calorimeter Heat Capacity In Chemistry.
From www.animalia-life.club
Calorimeter Diagram Calorimeter Heat Capacity In Chemistry The temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced by the. Learn how to calculate and interpret heat and related properties using typical calorimetry data. Calorimeters play a crucial role. Importance of calorimeters in chemistry. A calorimeter is a device used to measure the amount of heat. Calorimeter Heat Capacity In Chemistry.
From www.chegg.com
Solved Calculate the heat capacity of the calorimeter.Heat Calorimeter Heat Capacity In Chemistry Find out the definitions and. Apply the first law of thermodynamics to calorimetry. On the left (a), labeled ‘system exothermic process’, a calorimeter contains a substance that, during an exothermic reaction, releases heat. For example, when an exothermic. The temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced. Calorimeter Heat Capacity In Chemistry.
From www.fountainheadpress.com
Chemistry Illustrations Calorimeter Heat Capacity In Chemistry Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the chemical reaction. Importance of calorimeters in chemistry. Find out the definitions and. The temperature increase is measured and, along with. Calorimeter Heat Capacity In Chemistry.
From www.youtube.com
CHEMISTRY 101 Specific heat capacity and calculating heat YouTube Calorimeter Heat Capacity In Chemistry Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. Learn how to calculate and interpret heat and related properties using typical calorimetry data. Calorimeters play a crucial role. For example, when an exothermic. Apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus. Calorimeter Heat Capacity In Chemistry.
From wisc.pb.unizin.org
5.2 Calorimetry Chemistry Calorimeter Heat Capacity In Chemistry Importance of calorimeters in chemistry. Find out the definitions and. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Learn how to calculate and interpret heat and related properties using typical calorimetry data. Calorimeters play. Calorimeter Heat Capacity In Chemistry.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Calorimeter Heat Capacity In Chemistry The temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced by the. The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the chemical reaction. Learn how to calculate and interpret heat and related. Calorimeter Heat Capacity In Chemistry.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Calorimeter Heat Capacity In Chemistry Calorimeters play a crucial role. Importance of calorimeters in chemistry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Find out the definitions and. Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. Learn how to calculate and interpret. Calorimeter Heat Capacity In Chemistry.
From dokumen.tips
(PPTX) Calorimetry Calorimetry is used to measure heat capacity and Calorimeter Heat Capacity In Chemistry Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Importance of calorimeters in chemistry. On the left (a), labeled ‘system exothermic process’, a calorimeter contains a substance that, during an exothermic reaction, releases heat. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Apply. Calorimeter Heat Capacity In Chemistry.
From www.slideserve.com
PPT CHEM 1011 PowerPoint Presentation, free download ID317228 Calorimeter Heat Capacity In Chemistry Learn how to calculate and interpret heat and related properties using typical calorimetry data. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. On the left (a), labeled ‘system exothermic process’, a calorimeter contains a substance that, during an exothermic reaction, releases heat. Calorimeters are used to measure the heat of combustion, heat capacity,. Calorimeter Heat Capacity In Chemistry.
From learningcampusscarf.z13.web.core.windows.net
How To Calculate Heat Of Reaction Calorimetry Calorimeter Heat Capacity In Chemistry Learn how to calculate and interpret heat and related properties using typical calorimetry data. For example, when an exothermic. Find out the definitions and. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeters play. Calorimeter Heat Capacity In Chemistry.
From www.chegg.com
Solved CHEMISTRY DETERMINING HEAT CAPACITY OF A CALORIMETER Calorimeter Heat Capacity In Chemistry On the left (a), labeled ‘system exothermic process’, a calorimeter contains a substance that, during an exothermic reaction, releases heat. Importance of calorimeters in chemistry. Calorimeters play a crucial role. Learn how to calculate and interpret heat and related properties using typical calorimetry data. Apply the first law of thermodynamics to calorimetry. A calorimeter is a device used to measure. Calorimeter Heat Capacity In Chemistry.
From studylib.net
Calorimetry Lab Specific Heat Capacity Calorimeter Heat Capacity In Chemistry For example, when an exothermic. On the left (a), labeled ‘system exothermic process’, a calorimeter contains a substance that, during an exothermic reaction, releases heat. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Learn how to calculate and interpret heat and related properties using typical calorimetry data. The heat capacity of the calorimeter. Calorimeter Heat Capacity In Chemistry.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimeter Heat Capacity In Chemistry Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Find out the definitions and. Importance of calorimeters in chemistry. Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. Calorimeters. Calorimeter Heat Capacity In Chemistry.
From askfilo.com
In a constant pressure calorimeter with heat capacity of 453 Jk−1,200 mL Calorimeter Heat Capacity In Chemistry Learn how to calculate and interpret heat and related properties using typical calorimetry data. Calorimeters play a crucial role. Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. Apply the first law of thermodynamics to calorimetry. The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount. Calorimeter Heat Capacity In Chemistry.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Calorimeter Heat Capacity In Chemistry The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the chemical reaction. On the left (a), labeled ‘system exothermic process’, a calorimeter contains a substance that, during an exothermic reaction, releases heat. Apply the first law of thermodynamics to calorimetry. Calorimeters play a crucial role.. Calorimeter Heat Capacity In Chemistry.