Standard Heat Of Formation Of Br2 . The symbol for the standard enthalpy of formation is: All chemical reactions involve a change in enthalpy (defined as the heat produced or. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. The standard state heat of formation for the elemental form of each atom is zero. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including.
from studylib.net
Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. The symbol for the standard enthalpy of formation is: 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. All chemical reactions involve a change in enthalpy (defined as the heat produced or. The standard state heat of formation for the elemental form of each atom is zero. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and.
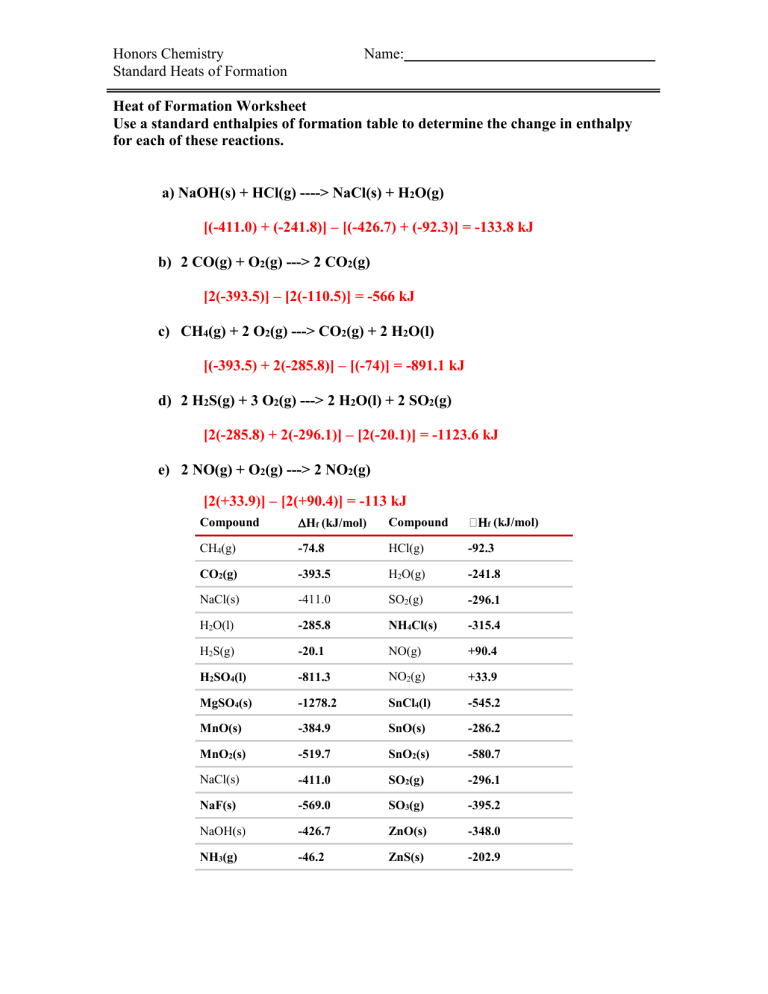
heats of formation worksheet key
Standard Heat Of Formation Of Br2 Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. The standard state heat of formation for the elemental form of each atom is zero. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. All chemical reactions involve a change in enthalpy (defined as the heat produced or. The symbol for the standard enthalpy of formation is:
From rayb78.github.io
Heat Of Formation Chart Standard Heat Of Formation Of Br2 Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard state heat of formation for the elemental form of each atom is zero. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The. Standard Heat Of Formation Of Br2.
From www.slideserve.com
PPT THERMOCHEMISTRY PowerPoint Presentation, free download ID4499046 Standard Heat Of Formation Of Br2 Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard state heat of formation for the elemental form of each atom is zero. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. The standard enthalpy of formation. Standard Heat Of Formation Of Br2.
From www.toppr.com
Scotopic movement induced by Biology Questions Standard Heat Of Formation Of Br2 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard state heat of formation for the elemental form of each atom is zero. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. The. Standard Heat Of Formation Of Br2.
From www.chegg.com
Solved (a) Write the balanced chemical equation that Standard Heat Of Formation Of Br2 All chemical reactions involve a change in enthalpy (defined as the heat produced or. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard state heat of formation for the elemental form of each atom is zero. Definition and explanation of the terms standard. Standard Heat Of Formation Of Br2.
From www.youtube.com
Standard heat of formation problem / Heat of formation formation Standard Heat Of Formation Of Br2 The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. All chemical reactions involve a change in enthalpy (defined as the heat produced or. Definition and explanation. Standard Heat Of Formation Of Br2.
From askfilo.com
Consider the following. (a) Standard enthalpy of formation of Br2 (I) is Standard Heat Of Formation Of Br2 The symbol for the standard enthalpy of formation is: All chemical reactions involve a change in enthalpy (defined as the heat produced or. The standard state heat of formation for the elemental form of each atom is zero. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. Standard Heat Of Formation Of Br2.
From www.chegg.com
Solved Select the product(s) for the following reaction. Br2 Standard Heat Of Formation Of Br2 Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard state heat of formation for the elemental form of each atom is zero. The symbol for the standard enthalpy of formation is: All chemical reactions involve a change in enthalpy (defined as the heat produced or. Definition. Standard Heat Of Formation Of Br2.
From www.numerade.com
SOLVED Use the following information to calculate the electron Standard Heat Of Formation Of Br2 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from. Standard Heat Of Formation Of Br2.
From www.coursehero.com
[Solved] 1. All of the following compounds have a standard heat of Standard Heat Of Formation Of Br2 All chemical reactions involve a change in enthalpy (defined as the heat produced or. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. The standard state heat of formation for the elemental form of each atom is zero. 193 rows in chemistry and thermodynamics, the standard enthalpy of. Standard Heat Of Formation Of Br2.
From lessonluft.z19.web.core.windows.net
Heat Of Formation Chart Standard Heat Of Formation Of Br2 All chemical reactions involve a change in enthalpy (defined as the heat produced or. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. 193 rows in chemistry and thermodynamics,. Standard Heat Of Formation Of Br2.
From studylib.net
heats of formation worksheet key Standard Heat Of Formation Of Br2 The symbol for the standard enthalpy of formation is: 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. The standard enthalpy of formation is a. Standard Heat Of Formation Of Br2.
From worksheetmagicadrienn.z21.web.core.windows.net
How To Calculate The Heat Of Formation Standard Heat Of Formation Of Br2 Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from. Standard Heat Of Formation Of Br2.
From www.numerade.com
SOLVED The standard enthalpy of formation for the reaction below is Standard Heat Of Formation Of Br2 This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. The standard state heat of formation for the elemental form of each atom is zero. The standard enthalpy. Standard Heat Of Formation Of Br2.
From www.slideserve.com
PPT Standard Heats of Reaction PowerPoint Presentation, free download Standard Heat Of Formation Of Br2 The standard state heat of formation for the elemental form of each atom is zero. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard.. Standard Heat Of Formation Of Br2.
From classschoolcole.z21.web.core.windows.net
How To Determine The Heat Of Formation Standard Heat Of Formation Of Br2 The symbol for the standard enthalpy of formation is: This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Definition and explanation of. Standard Heat Of Formation Of Br2.
From www.masterorganicchemistry.com
More On 1,2 and 1,4 Additions To Dienes Master Organic Chemistry Standard Heat Of Formation Of Br2 This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound. Standard Heat Of Formation Of Br2.
From darkataxa.blogspot.com
Astounding Collections Of Heat Of Formation Table Photos Darkata Standard Heat Of Formation Of Br2 The standard state heat of formation for the elemental form of each atom is zero. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. The symbol for the standard enthalpy of formation is: All chemical reactions involve a change in enthalpy (defined as the heat produced. Standard Heat Of Formation Of Br2.
From duanerafanan.blogspot.com
DUANE HESS'S LAW Standard Heat Of Formation Of Br2 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard state heat of formation for the elemental form of each atom. Standard Heat Of Formation Of Br2.
From brunofuga.adv.br
Standard Enthalpy Of Formation Definition, Table, Equation, 46 OFF Standard Heat Of Formation Of Br2 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard state heat of formation for the elemental form of each atom is zero. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and. Standard Heat Of Formation Of Br2.
From www.youtube.com
Br2 (Bromine gas) Molecular Geometry, Bond Angles YouTube Standard Heat Of Formation Of Br2 The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for. Standard Heat Of Formation Of Br2.
From www.numerade.com
SOLVED Question No 5 Timer 0119 Mark 2 If the standard enthalpies Standard Heat Of Formation Of Br2 The standard state heat of formation for the elemental form of each atom is zero. All chemical reactions involve a change in enthalpy (defined as the heat produced or. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The enthalpy of formation (\(δh_{f}\)) is the enthalpy. Standard Heat Of Formation Of Br2.
From rayb78.github.io
Heat Of Formation Chart Standard Heat Of Formation Of Br2 All chemical reactions involve a change in enthalpy (defined as the heat produced or. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change. Standard Heat Of Formation Of Br2.
From www.numerade.com
Construct an enthalpy diagram with the following data to calculate the Standard Heat Of Formation Of Br2 Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. All chemical reactions involve a change in enthalpy (defined as the heat produced or. The standard state heat of formation for the elemental form of each atom is zero. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that. Standard Heat Of Formation Of Br2.
From www.chegg.com
Solved Ph Br2 light (racemic) Br NBS heat Br2 light Standard Heat Of Formation Of Br2 The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The symbol for the standard enthalpy of formation is: 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. All chemical reactions involve. Standard Heat Of Formation Of Br2.
From byjus.com
The standard molar heat for formation ofethane, carbondioxide and water Standard Heat Of Formation Of Br2 The standard state heat of formation for the elemental form of each atom is zero. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. All. Standard Heat Of Formation Of Br2.
From www.showme.com
Standard heat of formation Science, Chemistry, thermochemistry ShowMe Standard Heat Of Formation Of Br2 Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from. Standard Heat Of Formation Of Br2.
From www.slideserve.com
PPT Formation Reactions PowerPoint Presentation, free download ID Standard Heat Of Formation Of Br2 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in. Standard Heat Of Formation Of Br2.
From www.youtube.com
Alkene + Br2 + H2O YouTube Standard Heat Of Formation Of Br2 All chemical reactions involve a change in enthalpy (defined as the heat produced or. The symbol for the standard enthalpy of formation is: Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound. Standard Heat Of Formation Of Br2.
From www.chegg.com
Solved 19. Use the given information to calculate the Standard Heat Of Formation Of Br2 The standard state heat of formation for the elemental form of each atom is zero. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. This table lists. Standard Heat Of Formation Of Br2.
From www.slideserve.com
PPT Standard Heats of Reaction PowerPoint Presentation, free download Standard Heat Of Formation Of Br2 This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of. Standard Heat Of Formation Of Br2.
From www.youtube.com
Br2 Lewis Structure How to Draw the Lewis Dot Structure for Dibromine Standard Heat Of Formation Of Br2 The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. The standard state heat of formation for the elemental form of each atom is zero. All chemical reactions involve a change in enthalpy (defined as the heat produced or. The standard enthalpy of formation is a measure of the energy released. Standard Heat Of Formation Of Br2.
From quizzschoolunderbuys.z13.web.core.windows.net
How To Determine The Heat Of Formation Standard Heat Of Formation Of Br2 Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound. Standard Heat Of Formation Of Br2.
From www.slideserve.com
PPT Chemistry 17.4 PowerPoint Presentation, free download ID2772524 Standard Heat Of Formation Of Br2 Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard. Standard Heat Of Formation Of Br2.
From www.chegg.com
Solved 5. Use standard heats of formation (table on last Standard Heat Of Formation Of Br2 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation. Standard Heat Of Formation Of Br2.
From mavink.com
Enthalpy Of Formation Symbol Standard Heat Of Formation Of Br2 The symbol for the standard enthalpy of formation is: The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard state heat of formation for the elemental form of. Standard Heat Of Formation Of Br2.