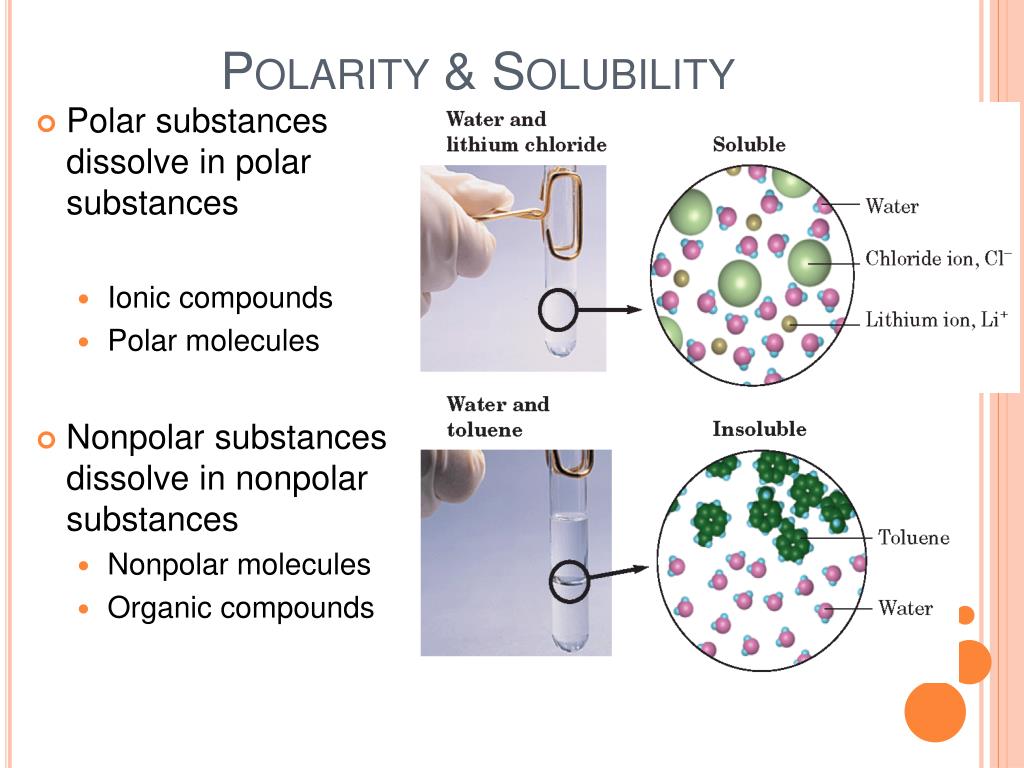
What Substances Dissolve In Water Polar Or Nonpolar . A bond between two or more atoms is. What makes a bond polar? Methanol (ch 3 oh) sodium sulfate (na 2 so 4) octane (c. If there is a portion of the molecule that is nonpolar, this nonpolar section interferes with the water molecules’ ability to form hydrogen. Cesium is the least electronegative element with an electronegativity of 0.7. Some substances comprised of nonpolar molecules will dissolve in water, but only to a limited degree. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. The rule of thumb is that “like dissolves like.” what this means is that polar solvents dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes. In this explainer, we will learn how to describe polar and nonpolar solvents. Which substances should dissolve in water? A polar bond is a type of covalent bond. Have you ever wondered why. Solubility can be defined as the tendency of one chemical. Water is considered a polar solvent.
from www.slideserve.com
Methanol (ch 3 oh) sodium sulfate (na 2 so 4) octane (c. Water is considered a polar solvent. The rule of thumb is that “like dissolves like.” what this means is that polar solvents dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes. A bond between two or more atoms is. Some substances comprised of nonpolar molecules will dissolve in water, but only to a limited degree. Which substances should dissolve in water? Cesium is the least electronegative element with an electronegativity of 0.7. What makes a bond polar? A polar bond is a type of covalent bond. Have you ever wondered why.
PPT Chapter 12 SOLUTIONS PowerPoint Presentation, free download ID
What Substances Dissolve In Water Polar Or Nonpolar If there is a portion of the molecule that is nonpolar, this nonpolar section interferes with the water molecules’ ability to form hydrogen. A polar bond is a type of covalent bond. What makes a bond polar? Which substances should dissolve in water? Some substances comprised of nonpolar molecules will dissolve in water, but only to a limited degree. Methanol (ch 3 oh) sodium sulfate (na 2 so 4) octane (c. If there is a portion of the molecule that is nonpolar, this nonpolar section interferes with the water molecules’ ability to form hydrogen. Cesium is the least electronegative element with an electronegativity of 0.7. Solubility can be defined as the tendency of one chemical. Have you ever wondered why. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. Water is considered a polar solvent. The rule of thumb is that “like dissolves like.” what this means is that polar solvents dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes. In this explainer, we will learn how to describe polar and nonpolar solvents. A bond between two or more atoms is.
From www.slideserve.com
PPT Chapter 2 Water PowerPoint Presentation, free download ID262198 What Substances Dissolve In Water Polar Or Nonpolar The rule of thumb is that “like dissolves like.” what this means is that polar solvents dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes. Have you ever wondered why. A bond between two or more atoms is. If there is a portion of the molecule that is nonpolar, this nonpolar section interferes with the water molecules’ ability to form. What Substances Dissolve In Water Polar Or Nonpolar.
From www.slideserve.com
PPT Chemistry of Life PowerPoint Presentation ID5875746 What Substances Dissolve In Water Polar Or Nonpolar Which substances should dissolve in water? The rule of thumb is that “like dissolves like.” what this means is that polar solvents dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes. A polar bond is a type of covalent bond. Methanol (ch 3 oh) sodium sulfate (na 2 so 4) octane (c. Solubility can be defined as the tendency of. What Substances Dissolve In Water Polar Or Nonpolar.
From www.expii.com
Polar vs. Nonpolar Bonds — Overview & Examples Expii What Substances Dissolve In Water Polar Or Nonpolar Some substances comprised of nonpolar molecules will dissolve in water, but only to a limited degree. A bond between two or more atoms is. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. What makes a bond polar? Methanol (ch 3 oh) sodium sulfate (na 2 so 4). What Substances Dissolve In Water Polar Or Nonpolar.
From www.slideserve.com
PPT Chapter 12 SOLUTIONS PowerPoint Presentation, free download ID What Substances Dissolve In Water Polar Or Nonpolar Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. Methanol (ch 3 oh) sodium sulfate (na 2 so 4) octane (c. Water is considered a polar solvent. In this explainer, we will learn how to describe polar and nonpolar solvents. Cesium is the least electronegative element with an. What Substances Dissolve In Water Polar Or Nonpolar.
From www.slideshare.net
Factors Affecting Solubility What Substances Dissolve In Water Polar Or Nonpolar If there is a portion of the molecule that is nonpolar, this nonpolar section interferes with the water molecules’ ability to form hydrogen. The rule of thumb is that “like dissolves like.” what this means is that polar solvents dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes. A bond between two or more atoms is. What makes a bond. What Substances Dissolve In Water Polar Or Nonpolar.
From www.slideserve.com
PPT Chapter 8 SOLUTIONS PowerPoint Presentation, free download ID What Substances Dissolve In Water Polar Or Nonpolar If there is a portion of the molecule that is nonpolar, this nonpolar section interferes with the water molecules’ ability to form hydrogen. Which substances should dissolve in water? Methanol (ch 3 oh) sodium sulfate (na 2 so 4) octane (c. Cesium is the least electronegative element with an electronegativity of 0.7. What makes a bond polar? In this explainer,. What Substances Dissolve In Water Polar Or Nonpolar.
From alden-yersblogweiss.blogspot.com
Describe Polar Covalent Bonds Using Water as an Example What Substances Dissolve In Water Polar Or Nonpolar In this explainer, we will learn how to describe polar and nonpolar solvents. The rule of thumb is that “like dissolves like.” what this means is that polar solvents dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes. Which substances should dissolve in water? Cesium is the least electronegative element with an electronegativity of 0.7. Some substances comprised of nonpolar. What Substances Dissolve In Water Polar Or Nonpolar.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID269800 What Substances Dissolve In Water Polar Or Nonpolar Solubility can be defined as the tendency of one chemical. If there is a portion of the molecule that is nonpolar, this nonpolar section interferes with the water molecules’ ability to form hydrogen. A polar bond is a type of covalent bond. Water is considered a polar solvent. Have you ever wondered why. Methanol (ch 3 oh) sodium sulfate (na. What Substances Dissolve In Water Polar Or Nonpolar.
From www.slideserve.com
PPT Solutions and Mixtures PowerPoint Presentation, free download What Substances Dissolve In Water Polar Or Nonpolar A bond between two or more atoms is. A polar bond is a type of covalent bond. Some substances comprised of nonpolar molecules will dissolve in water, but only to a limited degree. Methanol (ch 3 oh) sodium sulfate (na 2 so 4) octane (c. Cesium is the least electronegative element with an electronegativity of 0.7. What makes a bond. What Substances Dissolve In Water Polar Or Nonpolar.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation ID2276550 What Substances Dissolve In Water Polar Or Nonpolar Some substances comprised of nonpolar molecules will dissolve in water, but only to a limited degree. Which substances should dissolve in water? The rule of thumb is that “like dissolves like.” what this means is that polar solvents dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes. In this explainer, we will learn how to describe polar and nonpolar solvents.. What Substances Dissolve In Water Polar Or Nonpolar.
From sciencenotes.org
Why Is Water Called the Universal Solvent? What Substances Dissolve In Water Polar Or Nonpolar Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. A polar bond is a type of covalent bond. Which substances should dissolve in water? Cesium is the least electronegative element with an electronegativity of 0.7. Some substances comprised of nonpolar molecules will dissolve in water, but only to. What Substances Dissolve In Water Polar Or Nonpolar.
From studylib.net
Polar and Nonpolar Molecules What Substances Dissolve In Water Polar Or Nonpolar A polar bond is a type of covalent bond. If there is a portion of the molecule that is nonpolar, this nonpolar section interferes with the water molecules’ ability to form hydrogen. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. Water is considered a polar solvent. Which. What Substances Dissolve In Water Polar Or Nonpolar.
From www.slideserve.com
PPT CHAPTER 2 Small Molecules Structure and Behavior PowerPoint What Substances Dissolve In Water Polar Or Nonpolar Methanol (ch 3 oh) sodium sulfate (na 2 so 4) octane (c. Cesium is the least electronegative element with an electronegativity of 0.7. What makes a bond polar? If there is a portion of the molecule that is nonpolar, this nonpolar section interferes with the water molecules’ ability to form hydrogen. Water is considered a polar solvent. The rule of. What Substances Dissolve In Water Polar Or Nonpolar.
From www.slideserve.com
PPT Bonding PowerPoint Presentation ID3050946 What Substances Dissolve In Water Polar Or Nonpolar A polar bond is a type of covalent bond. Water is considered a polar solvent. In this explainer, we will learn how to describe polar and nonpolar solvents. Solubility can be defined as the tendency of one chemical. If there is a portion of the molecule that is nonpolar, this nonpolar section interferes with the water molecules’ ability to form. What Substances Dissolve In Water Polar Or Nonpolar.
From www.slideshare.net
Podcastppt5 What Substances Dissolve In Water Polar Or Nonpolar Methanol (ch 3 oh) sodium sulfate (na 2 so 4) octane (c. If there is a portion of the molecule that is nonpolar, this nonpolar section interferes with the water molecules’ ability to form hydrogen. In this explainer, we will learn how to describe polar and nonpolar solvents. Water is considered a polar solvent. Water, which not only dissolves many. What Substances Dissolve In Water Polar Or Nonpolar.
From webmis.highland.cc.il.us
Aqueous Solutions What Substances Dissolve In Water Polar Or Nonpolar A polar bond is a type of covalent bond. In this explainer, we will learn how to describe polar and nonpolar solvents. Methanol (ch 3 oh) sodium sulfate (na 2 so 4) octane (c. Water is considered a polar solvent. What makes a bond polar? Water, which not only dissolves many compounds but also dissolves more substances than any other. What Substances Dissolve In Water Polar Or Nonpolar.
From socratic.org
What kinds of molecules are polar? + Example What Substances Dissolve In Water Polar Or Nonpolar Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. Solubility can be defined as the tendency of one chemical. Some substances comprised of nonpolar molecules will dissolve in water, but only to a limited degree. Have you ever wondered why. A bond between two or more atoms is.. What Substances Dissolve In Water Polar Or Nonpolar.
From draw-hub.blogspot.com
Do Polar Or Nonpolar Molecules Dissolve In Water Drawhub What Substances Dissolve In Water Polar Or Nonpolar Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. Solubility can be defined as the tendency of one chemical. What makes a bond polar? Methanol (ch 3 oh) sodium sulfate (na 2 so 4) octane (c. The rule of thumb is that “like dissolves like.” what this means. What Substances Dissolve In Water Polar Or Nonpolar.
From slideplayer.com
Properties of Water. ppt download What Substances Dissolve In Water Polar Or Nonpolar Methanol (ch 3 oh) sodium sulfate (na 2 so 4) octane (c. A polar bond is a type of covalent bond. A bond between two or more atoms is. Some substances comprised of nonpolar molecules will dissolve in water, but only to a limited degree. The rule of thumb is that “like dissolves like.” what this means is that polar. What Substances Dissolve In Water Polar Or Nonpolar.
From sciencenotes.org
Polar and Nonpolar Molecules What Substances Dissolve In Water Polar Or Nonpolar Have you ever wondered why. What makes a bond polar? If there is a portion of the molecule that is nonpolar, this nonpolar section interferes with the water molecules’ ability to form hydrogen. Some substances comprised of nonpolar molecules will dissolve in water, but only to a limited degree. Solubility can be defined as the tendency of one chemical. Water,. What Substances Dissolve In Water Polar Or Nonpolar.
From bayleegroconner.blogspot.com
Is Water Polar or Nonpolar BayleegroConner What Substances Dissolve In Water Polar Or Nonpolar Solubility can be defined as the tendency of one chemical. What makes a bond polar? Water is considered a polar solvent. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. If there is a portion of the molecule that is nonpolar, this nonpolar section interferes with the water. What Substances Dissolve In Water Polar Or Nonpolar.
From courses.lumenlearning.com
Reading Covalent Bonds Biology I What Substances Dissolve In Water Polar Or Nonpolar Some substances comprised of nonpolar molecules will dissolve in water, but only to a limited degree. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. Water is considered a polar solvent. Have you ever wondered why. What makes a bond polar? Solubility can be defined as the tendency. What Substances Dissolve In Water Polar Or Nonpolar.
From slideplayer.com
Why are we studying water? ppt download What Substances Dissolve In Water Polar Or Nonpolar Solubility can be defined as the tendency of one chemical. The rule of thumb is that “like dissolves like.” what this means is that polar solvents dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes. In this explainer, we will learn how to describe polar and nonpolar solvents. What makes a bond polar? Have you ever wondered why. Water is. What Substances Dissolve In Water Polar Or Nonpolar.
From www.slideserve.com
PPT Chapter 2 Chemistry PowerPoint Presentation, free What Substances Dissolve In Water Polar Or Nonpolar Methanol (ch 3 oh) sodium sulfate (na 2 so 4) octane (c. If there is a portion of the molecule that is nonpolar, this nonpolar section interferes with the water molecules’ ability to form hydrogen. A bond between two or more atoms is. Water is considered a polar solvent. Solubility can be defined as the tendency of one chemical. Which. What Substances Dissolve In Water Polar Or Nonpolar.
From studyloadstones.z21.web.core.windows.net
Why Does Nonpolar Dissolve Nonpolar What Substances Dissolve In Water Polar Or Nonpolar What makes a bond polar? A bond between two or more atoms is. Which substances should dissolve in water? Methanol (ch 3 oh) sodium sulfate (na 2 so 4) octane (c. Solubility can be defined as the tendency of one chemical. The rule of thumb is that “like dissolves like.” what this means is that polar solvents dissolve polar solutes,. What Substances Dissolve In Water Polar Or Nonpolar.
From slideplayer.com
KEY CONCEPT Water’s unique properties allow life to exist on Earth What Substances Dissolve In Water Polar Or Nonpolar Methanol (ch 3 oh) sodium sulfate (na 2 so 4) octane (c. Which substances should dissolve in water? A bond between two or more atoms is. What makes a bond polar? Cesium is the least electronegative element with an electronegativity of 0.7. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered. What Substances Dissolve In Water Polar Or Nonpolar.
From issacropellis.blogspot.com
Is Water Polar or Nonpolar IssacropEllis What Substances Dissolve In Water Polar Or Nonpolar Some substances comprised of nonpolar molecules will dissolve in water, but only to a limited degree. Cesium is the least electronegative element with an electronegativity of 0.7. Which substances should dissolve in water? A bond between two or more atoms is. Have you ever wondered why. Solubility can be defined as the tendency of one chemical. If there is a. What Substances Dissolve In Water Polar Or Nonpolar.
From slidetodoc.com
HOW AND WHY DO SUBSTANCES DISSOLVE IN WATER What Substances Dissolve In Water Polar Or Nonpolar Have you ever wondered why. If there is a portion of the molecule that is nonpolar, this nonpolar section interferes with the water molecules’ ability to form hydrogen. What makes a bond polar? A polar bond is a type of covalent bond. Some substances comprised of nonpolar molecules will dissolve in water, but only to a limited degree. Solubility can. What Substances Dissolve In Water Polar Or Nonpolar.
From biologydictionary.net
Polar Molecule Definition and Examples Biology Dictionary What Substances Dissolve In Water Polar Or Nonpolar Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered the universal solvent. The rule of thumb is that “like dissolves like.” what this means is that polar solvents dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes. Which substances should dissolve in water? Some substances comprised of nonpolar molecules will dissolve. What Substances Dissolve In Water Polar Or Nonpolar.
From stilleducation.com
Polar and Nonpolar Covalent Bonds Characteristics & Differences What Substances Dissolve In Water Polar Or Nonpolar Cesium is the least electronegative element with an electronegativity of 0.7. What makes a bond polar? Which substances should dissolve in water? The rule of thumb is that “like dissolves like.” what this means is that polar solvents dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes. Have you ever wondered why. Methanol (ch 3 oh) sodium sulfate (na 2. What Substances Dissolve In Water Polar Or Nonpolar.
From lessonlibunidealism.z13.web.core.windows.net
Water As A Universal Solvent Notes What Substances Dissolve In Water Polar Or Nonpolar Which substances should dissolve in water? If there is a portion of the molecule that is nonpolar, this nonpolar section interferes with the water molecules’ ability to form hydrogen. In this explainer, we will learn how to describe polar and nonpolar solvents. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is considered. What Substances Dissolve In Water Polar Or Nonpolar.
From www.greelane.com
Definición y ejemplos de un enlace polar en Química What Substances Dissolve In Water Polar Or Nonpolar What makes a bond polar? Water is considered a polar solvent. The rule of thumb is that “like dissolves like.” what this means is that polar solvents dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes. Have you ever wondered why. A bond between two or more atoms is. Some substances comprised of nonpolar molecules will dissolve in water, but. What Substances Dissolve In Water Polar Or Nonpolar.
From www.slideserve.com
PPT Marine Biology Lesson 3 PowerPoint Presentation, free download What Substances Dissolve In Water Polar Or Nonpolar Cesium is the least electronegative element with an electronegativity of 0.7. Solubility can be defined as the tendency of one chemical. Methanol (ch 3 oh) sodium sulfate (na 2 so 4) octane (c. Have you ever wondered why. The rule of thumb is that “like dissolves like.” what this means is that polar solvents dissolve polar solutes, while nonpolar solvents. What Substances Dissolve In Water Polar Or Nonpolar.
From www.slideserve.com
PPT Solutions and Mixtures PowerPoint Presentation, free download What Substances Dissolve In Water Polar Or Nonpolar What makes a bond polar? Cesium is the least electronegative element with an electronegativity of 0.7. Methanol (ch 3 oh) sodium sulfate (na 2 so 4) octane (c. A polar bond is a type of covalent bond. Some substances comprised of nonpolar molecules will dissolve in water, but only to a limited degree. Water is considered a polar solvent. A. What Substances Dissolve In Water Polar Or Nonpolar.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation, free download ID6635190 What Substances Dissolve In Water Polar Or Nonpolar A bond between two or more atoms is. In this explainer, we will learn how to describe polar and nonpolar solvents. The rule of thumb is that “like dissolves like.” what this means is that polar solvents dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes. What makes a bond polar? Which substances should dissolve in water? Solubility can be. What Substances Dissolve In Water Polar Or Nonpolar.