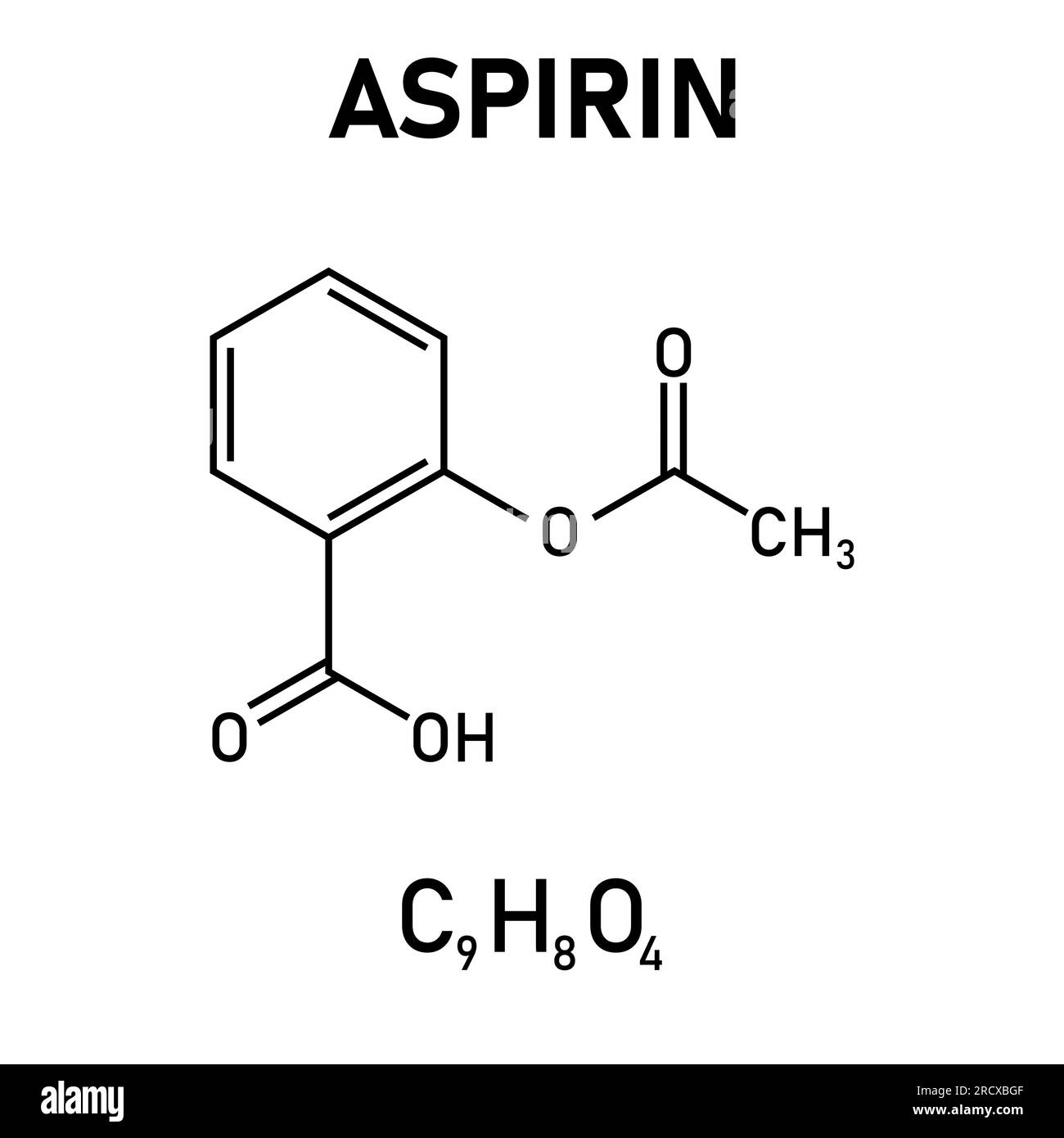
Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took . Aspirin binds to and acetylates serine (an amino acid used by the body to make proteins) residues in the active site of cyclooxygenase enzymes, leading to reduced production. Aspirin (acetylsalicylic acid, c9h8o4) is made by reacting salicylic acid (c7h6o3) with acetic anhydride [(ch3co)2o]: The molar mass of acetylsalicylic acid is approximately 179.97 g/mol. C 7 h 6 o 3 (s) + (c h 3. Describe the potential adverse effects of salicylic acid. Acetylsalicylic acid is a member of the class of benzoic acids that is salicylic acid in which the hydrogen that is attached to the phenolic hydroxy group has been replaced by an acetoxy group.
from www.alamy.com
Describe the potential adverse effects of salicylic acid. The molar mass of acetylsalicylic acid is approximately 179.97 g/mol. Aspirin (acetylsalicylic acid, c9h8o4) is made by reacting salicylic acid (c7h6o3) with acetic anhydride [(ch3co)2o]: Aspirin binds to and acetylates serine (an amino acid used by the body to make proteins) residues in the active site of cyclooxygenase enzymes, leading to reduced production. C 7 h 6 o 3 (s) + (c h 3. Acetylsalicylic acid is a member of the class of benzoic acids that is salicylic acid in which the hydrogen that is attached to the phenolic hydroxy group has been replaced by an acetoxy group.
Chemical structure of Aspirin or Acetylsalicylic acid (C9H8O4). Chemical resources for teachers
Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took C 7 h 6 o 3 (s) + (c h 3. Aspirin binds to and acetylates serine (an amino acid used by the body to make proteins) residues in the active site of cyclooxygenase enzymes, leading to reduced production. C 7 h 6 o 3 (s) + (c h 3. Describe the potential adverse effects of salicylic acid. The molar mass of acetylsalicylic acid is approximately 179.97 g/mol. Acetylsalicylic acid is a member of the class of benzoic acids that is salicylic acid in which the hydrogen that is attached to the phenolic hydroxy group has been replaced by an acetoxy group. Aspirin (acetylsalicylic acid, c9h8o4) is made by reacting salicylic acid (c7h6o3) with acetic anhydride [(ch3co)2o]:
From www.vrogue.co
Solved The Structure Of Aspirin Aka Acetylsalicylic A vrogue.co Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took Acetylsalicylic acid is a member of the class of benzoic acids that is salicylic acid in which the hydrogen that is attached to the phenolic hydroxy group has been replaced by an acetoxy group. The molar mass of acetylsalicylic acid is approximately 179.97 g/mol. Aspirin (acetylsalicylic acid, c9h8o4) is made by reacting salicylic acid (c7h6o3) with acetic anhydride [(ch3co)2o]: Describe. Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took.
From www.numerade.com
SOLVEDAn extrastrength aspirin tablet contains 0.500 g of the active ingredient Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took Acetylsalicylic acid is a member of the class of benzoic acids that is salicylic acid in which the hydrogen that is attached to the phenolic hydroxy group has been replaced by an acetoxy group. The molar mass of acetylsalicylic acid is approximately 179.97 g/mol. Describe the potential adverse effects of salicylic acid. C 7 h 6 o 3 (s) +. Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took.
From www.numerade.com
SOLVED The amount of active ingredient (acetylsalicylic acid, C9H8O4 ) per tablet of aspirin is Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took Aspirin (acetylsalicylic acid, c9h8o4) is made by reacting salicylic acid (c7h6o3) with acetic anhydride [(ch3co)2o]: The molar mass of acetylsalicylic acid is approximately 179.97 g/mol. Aspirin binds to and acetylates serine (an amino acid used by the body to make proteins) residues in the active site of cyclooxygenase enzymes, leading to reduced production. Acetylsalicylic acid is a member of the. Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took.
From www.freepik.com
Premium Photo Acetylsalicylic acid aspirin structural chemical formula Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took The molar mass of acetylsalicylic acid is approximately 179.97 g/mol. Aspirin (acetylsalicylic acid, c9h8o4) is made by reacting salicylic acid (c7h6o3) with acetic anhydride [(ch3co)2o]: C 7 h 6 o 3 (s) + (c h 3. Describe the potential adverse effects of salicylic acid. Acetylsalicylic acid is a member of the class of benzoic acids that is salicylic acid in. Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took.
From www.chegg.com
Solved 12. Acetylsalicylic acid, the active ingredient in Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took C 7 h 6 o 3 (s) + (c h 3. The molar mass of acetylsalicylic acid is approximately 179.97 g/mol. Aspirin binds to and acetylates serine (an amino acid used by the body to make proteins) residues in the active site of cyclooxygenase enzymes, leading to reduced production. Describe the potential adverse effects of salicylic acid. Aspirin (acetylsalicylic acid,. Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took.
From honeychempharma.com
Acetylsalicylic Acid(Aspirin) Honey Chem Pharmaceutical Research and Development Company Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took C 7 h 6 o 3 (s) + (c h 3. Aspirin (acetylsalicylic acid, c9h8o4) is made by reacting salicylic acid (c7h6o3) with acetic anhydride [(ch3co)2o]: Aspirin binds to and acetylates serine (an amino acid used by the body to make proteins) residues in the active site of cyclooxygenase enzymes, leading to reduced production. Acetylsalicylic acid is a member of. Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took.
From www.numerade.com
SOLVEDThe active ingredient in aspirin is acetyl salicylic acid with Ka=4.0 ×10^9 . The pH of Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took Aspirin (acetylsalicylic acid, c9h8o4) is made by reacting salicylic acid (c7h6o3) with acetic anhydride [(ch3co)2o]: Acetylsalicylic acid is a member of the class of benzoic acids that is salicylic acid in which the hydrogen that is attached to the phenolic hydroxy group has been replaced by an acetoxy group. The molar mass of acetylsalicylic acid is approximately 179.97 g/mol. Aspirin. Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took.
From dailymed.nlm.nih.gov
DailyMed ASPIRIN BOLUS acetylsalicylic acid tablet Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took Acetylsalicylic acid is a member of the class of benzoic acids that is salicylic acid in which the hydrogen that is attached to the phenolic hydroxy group has been replaced by an acetoxy group. Describe the potential adverse effects of salicylic acid. Aspirin binds to and acetylates serine (an amino acid used by the body to make proteins) residues in. Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took.
From studylib.net
Synthesis of acetylsalicylic acid (Aspirin) • Synthesis / working Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took Aspirin binds to and acetylates serine (an amino acid used by the body to make proteins) residues in the active site of cyclooxygenase enzymes, leading to reduced production. C 7 h 6 o 3 (s) + (c h 3. The molar mass of acetylsalicylic acid is approximately 179.97 g/mol. Describe the potential adverse effects of salicylic acid. Acetylsalicylic acid is. Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took.
From www.numerade.com
SOLVED Aspirin active ingredient is ASA, made from the following reaction Ifyou want 1000 Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took Aspirin binds to and acetylates serine (an amino acid used by the body to make proteins) residues in the active site of cyclooxygenase enzymes, leading to reduced production. C 7 h 6 o 3 (s) + (c h 3. The molar mass of acetylsalicylic acid is approximately 179.97 g/mol. Acetylsalicylic acid is a member of the class of benzoic acids. Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took.
From safrole.com
Acetylsalicylic Acid (Aspirin) Properties, Reactions and Applications Safrole Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took C 7 h 6 o 3 (s) + (c h 3. Aspirin (acetylsalicylic acid, c9h8o4) is made by reacting salicylic acid (c7h6o3) with acetic anhydride [(ch3co)2o]: Aspirin binds to and acetylates serine (an amino acid used by the body to make proteins) residues in the active site of cyclooxygenase enzymes, leading to reduced production. Describe the potential adverse effects of. Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took.
From www.alamy.com
Acetylsalicylic acid, aspirin, Structural chemical formula on a white background Stock Photo Alamy Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took Aspirin binds to and acetylates serine (an amino acid used by the body to make proteins) residues in the active site of cyclooxygenase enzymes, leading to reduced production. C 7 h 6 o 3 (s) + (c h 3. The molar mass of acetylsalicylic acid is approximately 179.97 g/mol. Aspirin (acetylsalicylic acid, c9h8o4) is made by reacting salicylic acid (c7h6o3). Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took.
From www.numerade.com
SOLVEDThe active ingredient in aspirin is acetylsalicylic acid. A 2.5 1g sample of Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took Describe the potential adverse effects of salicylic acid. The molar mass of acetylsalicylic acid is approximately 179.97 g/mol. Aspirin binds to and acetylates serine (an amino acid used by the body to make proteins) residues in the active site of cyclooxygenase enzymes, leading to reduced production. Aspirin (acetylsalicylic acid, c9h8o4) is made by reacting salicylic acid (c7h6o3) with acetic anhydride. Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took.
From www.numerade.com
SOLVED Consider acetylsalicylic acid (HC9H7O4; molar mass 180.16 g/mol), an active ingredient Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took C 7 h 6 o 3 (s) + (c h 3. Describe the potential adverse effects of salicylic acid. Aspirin binds to and acetylates serine (an amino acid used by the body to make proteins) residues in the active site of cyclooxygenase enzymes, leading to reduced production. Acetylsalicylic acid is a member of the class of benzoic acids that is. Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took.
From www.researchgate.net
Chemical structures of acetylsalicylic acid (aspirin), 4acetoxybenzoic... Download Scientific Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took Describe the potential adverse effects of salicylic acid. The molar mass of acetylsalicylic acid is approximately 179.97 g/mol. C 7 h 6 o 3 (s) + (c h 3. Aspirin binds to and acetylates serine (an amino acid used by the body to make proteins) residues in the active site of cyclooxygenase enzymes, leading to reduced production. Aspirin (acetylsalicylic acid,. Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took.
From www.numerade.com
SOLVEDAcetylsalicylic acid is the active ingredient in aspirin. It took 35.17 mL of 0.5065 M Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took Aspirin binds to and acetylates serine (an amino acid used by the body to make proteins) residues in the active site of cyclooxygenase enzymes, leading to reduced production. Acetylsalicylic acid is a member of the class of benzoic acids that is salicylic acid in which the hydrogen that is attached to the phenolic hydroxy group has been replaced by an. Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took.
From www.youtube.com
Aspirin (Acetylsalicylic Acid) YouTube Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took Describe the potential adverse effects of salicylic acid. C 7 h 6 o 3 (s) + (c h 3. Aspirin binds to and acetylates serine (an amino acid used by the body to make proteins) residues in the active site of cyclooxygenase enzymes, leading to reduced production. Acetylsalicylic acid is a member of the class of benzoic acids that is. Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took.
From www.dreamstime.com
Acetylsalicylic Acid, Aspirin, ASA Molecule. it is Salicylate, Analgesic and Antipyretic Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took Describe the potential adverse effects of salicylic acid. Acetylsalicylic acid is a member of the class of benzoic acids that is salicylic acid in which the hydrogen that is attached to the phenolic hydroxy group has been replaced by an acetoxy group. Aspirin binds to and acetylates serine (an amino acid used by the body to make proteins) residues in. Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took.
From dailymed.nlm.nih.gov
DailyMed ACETYLSALICYLIC ACID COATED aspirin tablet Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took Aspirin (acetylsalicylic acid, c9h8o4) is made by reacting salicylic acid (c7h6o3) with acetic anhydride [(ch3co)2o]: The molar mass of acetylsalicylic acid is approximately 179.97 g/mol. Describe the potential adverse effects of salicylic acid. Acetylsalicylic acid is a member of the class of benzoic acids that is salicylic acid in which the hydrogen that is attached to the phenolic hydroxy group. Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took.
From www.alamy.com
Chemical structure of Aspirin or Acetylsalicylic acid (C9H8O4). Chemical resources for teachers Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took The molar mass of acetylsalicylic acid is approximately 179.97 g/mol. Aspirin binds to and acetylates serine (an amino acid used by the body to make proteins) residues in the active site of cyclooxygenase enzymes, leading to reduced production. Describe the potential adverse effects of salicylic acid. Acetylsalicylic acid is a member of the class of benzoic acids that is salicylic. Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took.
From www.chegg.com
Solved The active ingredient in aspirin is acetylsalicylic Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took Acetylsalicylic acid is a member of the class of benzoic acids that is salicylic acid in which the hydrogen that is attached to the phenolic hydroxy group has been replaced by an acetoxy group. Aspirin binds to and acetylates serine (an amino acid used by the body to make proteins) residues in the active site of cyclooxygenase enzymes, leading to. Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took.
From www.chegg.com
Solved The active ingredient in aspirin is acetylsalicylic Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took C 7 h 6 o 3 (s) + (c h 3. The molar mass of acetylsalicylic acid is approximately 179.97 g/mol. Acetylsalicylic acid is a member of the class of benzoic acids that is salicylic acid in which the hydrogen that is attached to the phenolic hydroxy group has been replaced by an acetoxy group. Describe the potential adverse effects. Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took.
From www.numerade.com
SOLVED Unit 6 Titration Practice Worksheet Answer the following questions about acetylsalicylic Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took Aspirin binds to and acetylates serine (an amino acid used by the body to make proteins) residues in the active site of cyclooxygenase enzymes, leading to reduced production. The molar mass of acetylsalicylic acid is approximately 179.97 g/mol. Acetylsalicylic acid is a member of the class of benzoic acids that is salicylic acid in which the hydrogen that is attached. Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took.
From www.chegg.com
Solved The active ingredient in aspirin is acetylsalicylic Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took C 7 h 6 o 3 (s) + (c h 3. Describe the potential adverse effects of salicylic acid. Aspirin binds to and acetylates serine (an amino acid used by the body to make proteins) residues in the active site of cyclooxygenase enzymes, leading to reduced production. Acetylsalicylic acid is a member of the class of benzoic acids that is. Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took.
From www.numerade.com
SOLVED The active ingredient in aspirin is acetylsalicylic acid (HC9H7O4), a monoprotic acid Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took Aspirin (acetylsalicylic acid, c9h8o4) is made by reacting salicylic acid (c7h6o3) with acetic anhydride [(ch3co)2o]: Aspirin binds to and acetylates serine (an amino acid used by the body to make proteins) residues in the active site of cyclooxygenase enzymes, leading to reduced production. C 7 h 6 o 3 (s) + (c h 3. Describe the potential adverse effects of. Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took.
From www.numerade.com
SOLVED The amount of active ingredient (acetylsalicylic acid, C9H8O4 ) per tablet of aspirin is Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took The molar mass of acetylsalicylic acid is approximately 179.97 g/mol. Aspirin binds to and acetylates serine (an amino acid used by the body to make proteins) residues in the active site of cyclooxygenase enzymes, leading to reduced production. Describe the potential adverse effects of salicylic acid. Aspirin (acetylsalicylic acid, c9h8o4) is made by reacting salicylic acid (c7h6o3) with acetic anhydride. Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took.
From www.alamy.com
Micropirin enteric coated tablets contain the active ingredient acetylsalicylic acid, otherwise Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took Describe the potential adverse effects of salicylic acid. The molar mass of acetylsalicylic acid is approximately 179.97 g/mol. Acetylsalicylic acid is a member of the class of benzoic acids that is salicylic acid in which the hydrogen that is attached to the phenolic hydroxy group has been replaced by an acetoxy group. C 7 h 6 o 3 (s) +. Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took.
From www.numerade.com
SOLVEDAcetylsalicylic acid (HC, H 7 O4 ) is a monoprotic acid commonly known as "aspirin." A Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took Describe the potential adverse effects of salicylic acid. Aspirin binds to and acetylates serine (an amino acid used by the body to make proteins) residues in the active site of cyclooxygenase enzymes, leading to reduced production. Acetylsalicylic acid is a member of the class of benzoic acids that is salicylic acid in which the hydrogen that is attached to the. Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took.
From www.toppr.com
The active ingredient in aspirin is acetyl salicylic acid with K_{a}=4.0 times 10^{9}. The pH Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took The molar mass of acetylsalicylic acid is approximately 179.97 g/mol. C 7 h 6 o 3 (s) + (c h 3. Acetylsalicylic acid is a member of the class of benzoic acids that is salicylic acid in which the hydrogen that is attached to the phenolic hydroxy group has been replaced by an acetoxy group. Aspirin (acetylsalicylic acid, c9h8o4) is. Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took.
From www.alamy.com
Acetylsalicylic acid, aspirin, ASA molecule. It is salicylate, analgesic and antipyretic Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took Describe the potential adverse effects of salicylic acid. Acetylsalicylic acid is a member of the class of benzoic acids that is salicylic acid in which the hydrogen that is attached to the phenolic hydroxy group has been replaced by an acetoxy group. The molar mass of acetylsalicylic acid is approximately 179.97 g/mol. Aspirin binds to and acetylates serine (an amino. Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took.
From www.chegg.com
Solved The active ingredient in aspirin is acetylsalicylic Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took Aspirin (acetylsalicylic acid, c9h8o4) is made by reacting salicylic acid (c7h6o3) with acetic anhydride [(ch3co)2o]: Acetylsalicylic acid is a member of the class of benzoic acids that is salicylic acid in which the hydrogen that is attached to the phenolic hydroxy group has been replaced by an acetoxy group. Describe the potential adverse effects of salicylic acid. Aspirin binds to. Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took.
From www.alamy.com
Acetylsalicylic acid, aspirin, ASA molecule. It is salicylate, analgesic and antipyretic Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took Aspirin (acetylsalicylic acid, c9h8o4) is made by reacting salicylic acid (c7h6o3) with acetic anhydride [(ch3co)2o]: The molar mass of acetylsalicylic acid is approximately 179.97 g/mol. Describe the potential adverse effects of salicylic acid. Acetylsalicylic acid is a member of the class of benzoic acids that is salicylic acid in which the hydrogen that is attached to the phenolic hydroxy group. Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took.
From www.numerade.com
SOLVED Lab 8 Spectrophotometric Analysis of Aspirin Introduction Acetylsalicylic acid, the Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took Aspirin binds to and acetylates serine (an amino acid used by the body to make proteins) residues in the active site of cyclooxygenase enzymes, leading to reduced production. C 7 h 6 o 3 (s) + (c h 3. The molar mass of acetylsalicylic acid is approximately 179.97 g/mol. Aspirin (acetylsalicylic acid, c9h8o4) is made by reacting salicylic acid (c7h6o3). Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took.
From www.dreamstime.com
Acetylsalicylic Acid, Aspirin, Structural Chemical Formula Stock Photo Image of acid Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took The molar mass of acetylsalicylic acid is approximately 179.97 g/mol. Acetylsalicylic acid is a member of the class of benzoic acids that is salicylic acid in which the hydrogen that is attached to the phenolic hydroxy group has been replaced by an acetoxy group. Aspirin binds to and acetylates serine (an amino acid used by the body to make proteins). Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took.
From time.news
These are the side effects of acetylsalicylic acid, the active ingredient in Aspirin Time News Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took C 7 h 6 o 3 (s) + (c h 3. Aspirin (acetylsalicylic acid, c9h8o4) is made by reacting salicylic acid (c7h6o3) with acetic anhydride [(ch3co)2o]: The molar mass of acetylsalicylic acid is approximately 179.97 g/mol. Aspirin binds to and acetylates serine (an amino acid used by the body to make proteins) residues in the active site of cyclooxygenase enzymes,. Acetylsalicylic Acid Is The Active Ingredient In Aspirin. It Took.