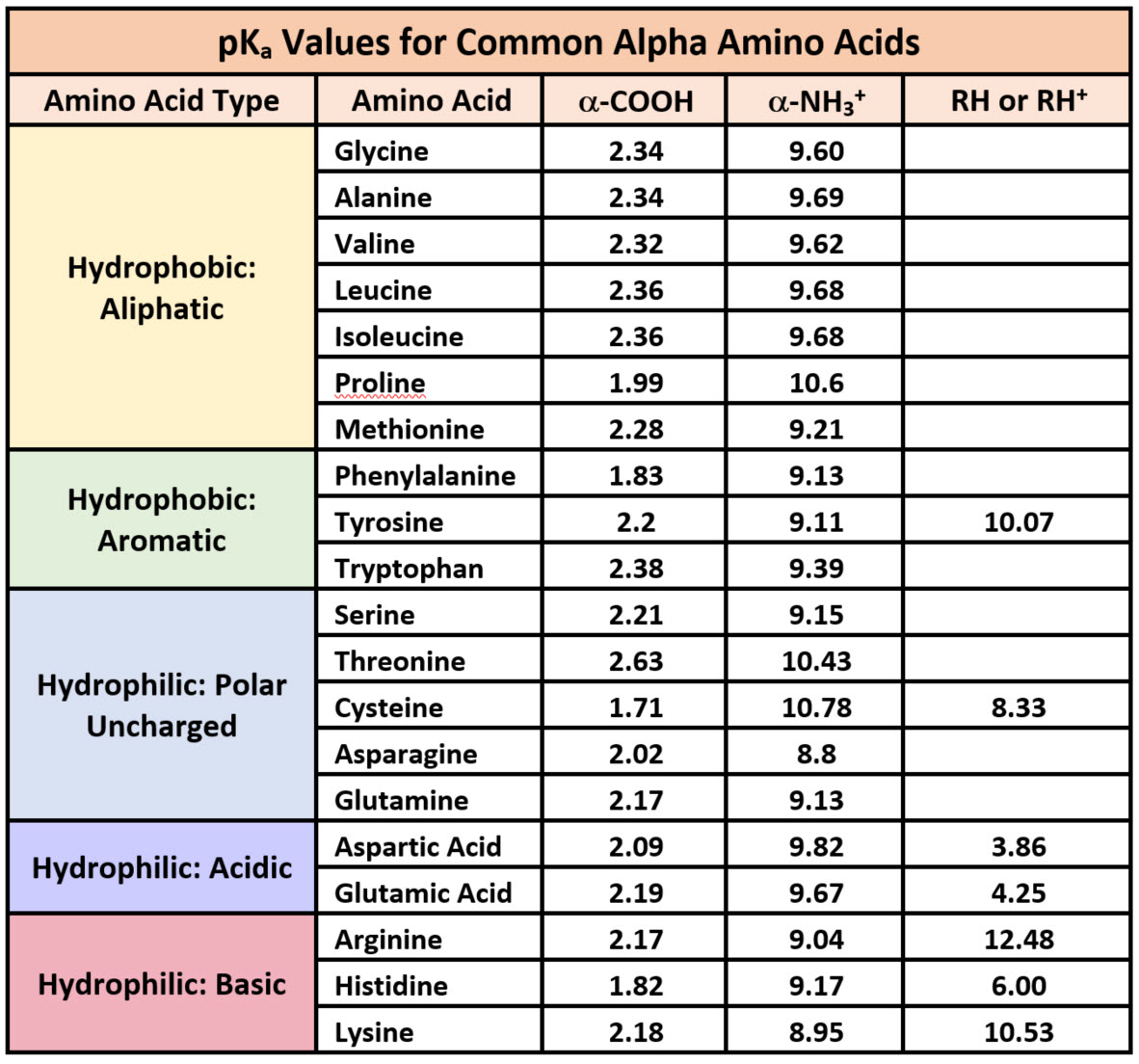
Amino Acid Carboxyl Group Pka . 22 rows table of pka and pi values. If you have a reaction where it looks like. At neutral ph the amino group is protonated, and the carboxyl group is. Why is pk a so important? It is readily produced by transamination of. Because every nucleophile is potentially a base, and vice versa. This carbon is designated as the α. For simple amino acids such as alanine, the pi is an average of the pk a 's of the carboxyl (2.34) and ammonium (9.69) groups. Thus, the pi for alanine is calculated to be: At a ph of 6.02, both the amino group and the carboxyl group are simultaneously protonated and deprotonated, leading to the formation of the. Each amino acid is structured from an amino group and a carboxyl group bound to a tetrahedral carbon.
from bio.libretexts.org
Because every nucleophile is potentially a base, and vice versa. This carbon is designated as the α. At neutral ph the amino group is protonated, and the carboxyl group is. If you have a reaction where it looks like. At a ph of 6.02, both the amino group and the carboxyl group are simultaneously protonated and deprotonated, leading to the formation of the. For simple amino acids such as alanine, the pi is an average of the pk a 's of the carboxyl (2.34) and ammonium (9.69) groups. 22 rows table of pka and pi values. Why is pk a so important? It is readily produced by transamination of. Each amino acid is structured from an amino group and a carboxyl group bound to a tetrahedral carbon.
3.1 Amino Acids and Peptides Biology LibreTexts
Amino Acid Carboxyl Group Pka If you have a reaction where it looks like. At neutral ph the amino group is protonated, and the carboxyl group is. It is readily produced by transamination of. At a ph of 6.02, both the amino group and the carboxyl group are simultaneously protonated and deprotonated, leading to the formation of the. If you have a reaction where it looks like. Why is pk a so important? Each amino acid is structured from an amino group and a carboxyl group bound to a tetrahedral carbon. Because every nucleophile is potentially a base, and vice versa. This carbon is designated as the α. 22 rows table of pka and pi values. For simple amino acids such as alanine, the pi is an average of the pk a 's of the carboxyl (2.34) and ammonium (9.69) groups. Thus, the pi for alanine is calculated to be:
From www.anyrgb.com
Pka, amino Acids, chem, Aspartic acid, leucine, reagent, acetic Acid Amino Acid Carboxyl Group Pka Because every nucleophile is potentially a base, and vice versa. For simple amino acids such as alanine, the pi is an average of the pk a 's of the carboxyl (2.34) and ammonium (9.69) groups. If you have a reaction where it looks like. Thus, the pi for alanine is calculated to be: 22 rows table of pka and pi. Amino Acid Carboxyl Group Pka.
From www.numerade.com
SOLVED Table 3.2 pKa Values of Common Amino Acids Acid aCOOH aNH3 Amino Acid Carboxyl Group Pka Because every nucleophile is potentially a base, and vice versa. This carbon is designated as the α. Each amino acid is structured from an amino group and a carboxyl group bound to a tetrahedral carbon. Thus, the pi for alanine is calculated to be: If you have a reaction where it looks like. At neutral ph the amino group is. Amino Acid Carboxyl Group Pka.
From www.animalia-life.club
Amino Group And Carboxyl Group Amino Acid Carboxyl Group Pka At a ph of 6.02, both the amino group and the carboxyl group are simultaneously protonated and deprotonated, leading to the formation of the. For simple amino acids such as alanine, the pi is an average of the pk a 's of the carboxyl (2.34) and ammonium (9.69) groups. Why is pk a so important? Thus, the pi for alanine. Amino Acid Carboxyl Group Pka.
From www.masterorganicchemistry.com
Isoelectric Points of Amino Acids (and How To Calculate Them) Master Amino Acid Carboxyl Group Pka Thus, the pi for alanine is calculated to be: Because every nucleophile is potentially a base, and vice versa. Why is pk a so important? If you have a reaction where it looks like. This carbon is designated as the α. Each amino acid is structured from an amino group and a carboxyl group bound to a tetrahedral carbon. 22. Amino Acid Carboxyl Group Pka.
From www.numerade.com
SOLVED The pKa values of the carboxyl groups of common amino acids are Amino Acid Carboxyl Group Pka At a ph of 6.02, both the amino group and the carboxyl group are simultaneously protonated and deprotonated, leading to the formation of the. Each amino acid is structured from an amino group and a carboxyl group bound to a tetrahedral carbon. This carbon is designated as the α. Why is pk a so important? If you have a reaction. Amino Acid Carboxyl Group Pka.
From www.organicchemistrytutor.com
AcidBase Equilibrium Part 1 How to Use the pKa Table — Organic Amino Acid Carboxyl Group Pka 22 rows table of pka and pi values. If you have a reaction where it looks like. Thus, the pi for alanine is calculated to be: For simple amino acids such as alanine, the pi is an average of the pk a 's of the carboxyl (2.34) and ammonium (9.69) groups. It is readily produced by transamination of. Each amino. Amino Acid Carboxyl Group Pka.
From www.numerade.com
SOLVED The pKa values of the carboxyl groups of common amino acids are Amino Acid Carboxyl Group Pka If you have a reaction where it looks like. Why is pk a so important? Thus, the pi for alanine is calculated to be: At a ph of 6.02, both the amino group and the carboxyl group are simultaneously protonated and deprotonated, leading to the formation of the. This carbon is designated as the α. Because every nucleophile is potentially. Amino Acid Carboxyl Group Pka.
From www.bartleby.com
Answered The chemical structure of an endogenous… bartleby Amino Acid Carboxyl Group Pka If you have a reaction where it looks like. For simple amino acids such as alanine, the pi is an average of the pk a 's of the carboxyl (2.34) and ammonium (9.69) groups. 22 rows table of pka and pi values. At neutral ph the amino group is protonated, and the carboxyl group is. Because every nucleophile is potentially. Amino Acid Carboxyl Group Pka.
From leah4sci.com
Amino Acid Charge in Zwitterions and Isoelectric Point MCAT Tutorial Amino Acid Carboxyl Group Pka It is readily produced by transamination of. At neutral ph the amino group is protonated, and the carboxyl group is. Each amino acid is structured from an amino group and a carboxyl group bound to a tetrahedral carbon. For simple amino acids such as alanine, the pi is an average of the pk a 's of the carboxyl (2.34) and. Amino Acid Carboxyl Group Pka.
From www.masterorganicchemistry.com
Simplifying the reactions of carboxylic acid derivatives (part 1 Amino Acid Carboxyl Group Pka If you have a reaction where it looks like. Thus, the pi for alanine is calculated to be: It is readily produced by transamination of. For simple amino acids such as alanine, the pi is an average of the pk a 's of the carboxyl (2.34) and ammonium (9.69) groups. 22 rows table of pka and pi values. Each amino. Amino Acid Carboxyl Group Pka.
From www.masterorganicchemistry.com
pKa Values Span 60 Orders Of Magnitude (!) Putting Them In Perspective Amino Acid Carboxyl Group Pka If you have a reaction where it looks like. At a ph of 6.02, both the amino group and the carboxyl group are simultaneously protonated and deprotonated, leading to the formation of the. For simple amino acids such as alanine, the pi is an average of the pk a 's of the carboxyl (2.34) and ammonium (9.69) groups. Why is. Amino Acid Carboxyl Group Pka.
From www.animalia-life.club
Amino Group And Carboxyl Group Amino Acid Carboxyl Group Pka It is readily produced by transamination of. Thus, the pi for alanine is calculated to be: If you have a reaction where it looks like. Because every nucleophile is potentially a base, and vice versa. At a ph of 6.02, both the amino group and the carboxyl group are simultaneously protonated and deprotonated, leading to the formation of the. This. Amino Acid Carboxyl Group Pka.
From www.chegg.com
Solved Table 1 pKa values of the 20 common amino acids. Amino Acid Carboxyl Group Pka It is readily produced by transamination of. Thus, the pi for alanine is calculated to be: 22 rows table of pka and pi values. At a ph of 6.02, both the amino group and the carboxyl group are simultaneously protonated and deprotonated, leading to the formation of the. This carbon is designated as the α. Because every nucleophile is potentially. Amino Acid Carboxyl Group Pka.
From www.masterorganicchemistry.com
AcidBase Reactions Introducing Ka and pKa Master Organic Chemistry Amino Acid Carboxyl Group Pka Each amino acid is structured from an amino group and a carboxyl group bound to a tetrahedral carbon. For simple amino acids such as alanine, the pi is an average of the pk a 's of the carboxyl (2.34) and ammonium (9.69) groups. 22 rows table of pka and pi values. It is readily produced by transamination of. Thus, the. Amino Acid Carboxyl Group Pka.
From www.pinterest.com
Pin on Biochemistry 2018 Amino Acid Carboxyl Group Pka Because every nucleophile is potentially a base, and vice versa. For simple amino acids such as alanine, the pi is an average of the pk a 's of the carboxyl (2.34) and ammonium (9.69) groups. At neutral ph the amino group is protonated, and the carboxyl group is. If you have a reaction where it looks like. This carbon is. Amino Acid Carboxyl Group Pka.
From www.numerade.com
SOLVED 8 The amino acid arginine (shown to the right) has the Amino Acid Carboxyl Group Pka At a ph of 6.02, both the amino group and the carboxyl group are simultaneously protonated and deprotonated, leading to the formation of the. It is readily produced by transamination of. 22 rows table of pka and pi values. For simple amino acids such as alanine, the pi is an average of the pk a 's of the carboxyl (2.34). Amino Acid Carboxyl Group Pka.
From www.reddit.com
Question on pKa values for amino acids r/Mcat Amino Acid Carboxyl Group Pka At a ph of 6.02, both the amino group and the carboxyl group are simultaneously protonated and deprotonated, leading to the formation of the. It is readily produced by transamination of. At neutral ph the amino group is protonated, and the carboxyl group is. Thus, the pi for alanine is calculated to be: 22 rows table of pka and pi. Amino Acid Carboxyl Group Pka.
From www.numerade.com
SOLVED TABLE 2.1 Typical pKa values of ionizable groups in prulci Acid Amino Acid Carboxyl Group Pka 22 rows table of pka and pi values. Because every nucleophile is potentially a base, and vice versa. Thus, the pi for alanine is calculated to be: If you have a reaction where it looks like. For simple amino acids such as alanine, the pi is an average of the pk a 's of the carboxyl (2.34) and ammonium (9.69). Amino Acid Carboxyl Group Pka.
From www.researchgate.net
Amino Acid pKa and corresponding protonation states at low, medium, and Amino Acid Carboxyl Group Pka Each amino acid is structured from an amino group and a carboxyl group bound to a tetrahedral carbon. At neutral ph the amino group is protonated, and the carboxyl group is. Why is pk a so important? 22 rows table of pka and pi values. Thus, the pi for alanine is calculated to be: Because every nucleophile is potentially a. Amino Acid Carboxyl Group Pka.
From www.chemistrysteps.com
Naming Carboxylic Acids Chemistry Steps Amino Acid Carboxyl Group Pka Thus, the pi for alanine is calculated to be: Why is pk a so important? Each amino acid is structured from an amino group and a carboxyl group bound to a tetrahedral carbon. 22 rows table of pka and pi values. This carbon is designated as the α. Because every nucleophile is potentially a base, and vice versa. At neutral. Amino Acid Carboxyl Group Pka.
From www.vrogue.co
Pka Of The Functional Groups Values To Know Pka Chart vrogue.co Amino Acid Carboxyl Group Pka Thus, the pi for alanine is calculated to be: Each amino acid is structured from an amino group and a carboxyl group bound to a tetrahedral carbon. 22 rows table of pka and pi values. At neutral ph the amino group is protonated, and the carboxyl group is. Why is pk a so important? It is readily produced by transamination. Amino Acid Carboxyl Group Pka.
From www.chegg.com
Solved 17. The pka for the carboxylic acid portion of the Amino Acid Carboxyl Group Pka At a ph of 6.02, both the amino group and the carboxyl group are simultaneously protonated and deprotonated, leading to the formation of the. For simple amino acids such as alanine, the pi is an average of the pk a 's of the carboxyl (2.34) and ammonium (9.69) groups. At neutral ph the amino group is protonated, and the carboxyl. Amino Acid Carboxyl Group Pka.
From www.vrogue.co
How To Use A Pka Table vrogue.co Amino Acid Carboxyl Group Pka Each amino acid is structured from an amino group and a carboxyl group bound to a tetrahedral carbon. For simple amino acids such as alanine, the pi is an average of the pk a 's of the carboxyl (2.34) and ammonium (9.69) groups. At neutral ph the amino group is protonated, and the carboxyl group is. It is readily produced. Amino Acid Carboxyl Group Pka.
From www.chemistrysteps.com
The pKa in Organic Chemistry Chemistry Steps Amino Acid Carboxyl Group Pka 22 rows table of pka and pi values. Because every nucleophile is potentially a base, and vice versa. Each amino acid is structured from an amino group and a carboxyl group bound to a tetrahedral carbon. If you have a reaction where it looks like. It is readily produced by transamination of. This carbon is designated as the α. Why. Amino Acid Carboxyl Group Pka.
From www.researchgate.net
Measured pKa values for selected BCAA/FBCAA pairs. pKa acid 5 Amino Acid Carboxyl Group Pka At neutral ph the amino group is protonated, and the carboxyl group is. Each amino acid is structured from an amino group and a carboxyl group bound to a tetrahedral carbon. It is readily produced by transamination of. 22 rows table of pka and pi values. For simple amino acids such as alanine, the pi is an average of the. Amino Acid Carboxyl Group Pka.
From mavink.com
Amino Acid Structure Labeled Amino Acid Carboxyl Group Pka Because every nucleophile is potentially a base, and vice versa. Each amino acid is structured from an amino group and a carboxyl group bound to a tetrahedral carbon. Why is pk a so important? For simple amino acids such as alanine, the pi is an average of the pk a 's of the carboxyl (2.34) and ammonium (9.69) groups. At. Amino Acid Carboxyl Group Pka.
From www.pinterest.com
amino acids with pKas of most Rgroups AND backbone groups Biology Amino Acid Carboxyl Group Pka Each amino acid is structured from an amino group and a carboxyl group bound to a tetrahedral carbon. 22 rows table of pka and pi values. Because every nucleophile is potentially a base, and vice versa. This carbon is designated as the α. At neutral ph the amino group is protonated, and the carboxyl group is. It is readily produced. Amino Acid Carboxyl Group Pka.
From www.masterorganicchemistry.com
How To Use a pKa Table Amino Acid Carboxyl Group Pka 22 rows table of pka and pi values. This carbon is designated as the α. Why is pk a so important? For simple amino acids such as alanine, the pi is an average of the pk a 's of the carboxyl (2.34) and ammonium (9.69) groups. It is readily produced by transamination of. If you have a reaction where it. Amino Acid Carboxyl Group Pka.
From www.numerade.com
Table 2 Typical pKa values of ionizable groups in proteins Group Acid Amino Acid Carboxyl Group Pka If you have a reaction where it looks like. For simple amino acids such as alanine, the pi is an average of the pk a 's of the carboxyl (2.34) and ammonium (9.69) groups. Why is pk a so important? Because every nucleophile is potentially a base, and vice versa. At neutral ph the amino group is protonated, and the. Amino Acid Carboxyl Group Pka.
From www.researchgate.net
4. Two amino acids (the carboxylicgroup from the amino acid 1 and the Amino Acid Carboxyl Group Pka For simple amino acids such as alanine, the pi is an average of the pk a 's of the carboxyl (2.34) and ammonium (9.69) groups. At a ph of 6.02, both the amino group and the carboxyl group are simultaneously protonated and deprotonated, leading to the formation of the. It is readily produced by transamination of. Why is pk a. Amino Acid Carboxyl Group Pka.
From www.numerade.com
SOLVED A new amino acid has been isolated that has been titrated to Amino Acid Carboxyl Group Pka 22 rows table of pka and pi values. Thus, the pi for alanine is calculated to be: Because every nucleophile is potentially a base, and vice versa. At neutral ph the amino group is protonated, and the carboxyl group is. If you have a reaction where it looks like. Each amino acid is structured from an amino group and a. Amino Acid Carboxyl Group Pka.
From kpu.pressbooks.pub
3.3 pKa of Organic Acids and Application of pKa to Predict AcidBase Amino Acid Carboxyl Group Pka It is readily produced by transamination of. Because every nucleophile is potentially a base, and vice versa. If you have a reaction where it looks like. At neutral ph the amino group is protonated, and the carboxyl group is. Thus, the pi for alanine is calculated to be: At a ph of 6.02, both the amino group and the carboxyl. Amino Acid Carboxyl Group Pka.
From bio.libretexts.org
3.1 Amino Acids and Peptides Biology LibreTexts Amino Acid Carboxyl Group Pka 22 rows table of pka and pi values. Thus, the pi for alanine is calculated to be: At neutral ph the amino group is protonated, and the carboxyl group is. Because every nucleophile is potentially a base, and vice versa. This carbon is designated as the α. If you have a reaction where it looks like. Each amino acid is. Amino Acid Carboxyl Group Pka.
From www.numerade.com
SOLVED You have 1 M(1 mol / L) solution of an amino acid. This amino Amino Acid Carboxyl Group Pka Because every nucleophile is potentially a base, and vice versa. If you have a reaction where it looks like. Each amino acid is structured from an amino group and a carboxyl group bound to a tetrahedral carbon. At a ph of 6.02, both the amino group and the carboxyl group are simultaneously protonated and deprotonated, leading to the formation of. Amino Acid Carboxyl Group Pka.
From www.numerade.com
SOLVED All amino acids have two ionizable functional groups an α Amino Acid Carboxyl Group Pka Thus, the pi for alanine is calculated to be: Why is pk a so important? At neutral ph the amino group is protonated, and the carboxyl group is. Because every nucleophile is potentially a base, and vice versa. If you have a reaction where it looks like. It is readily produced by transamination of. For simple amino acids such as. Amino Acid Carboxyl Group Pka.