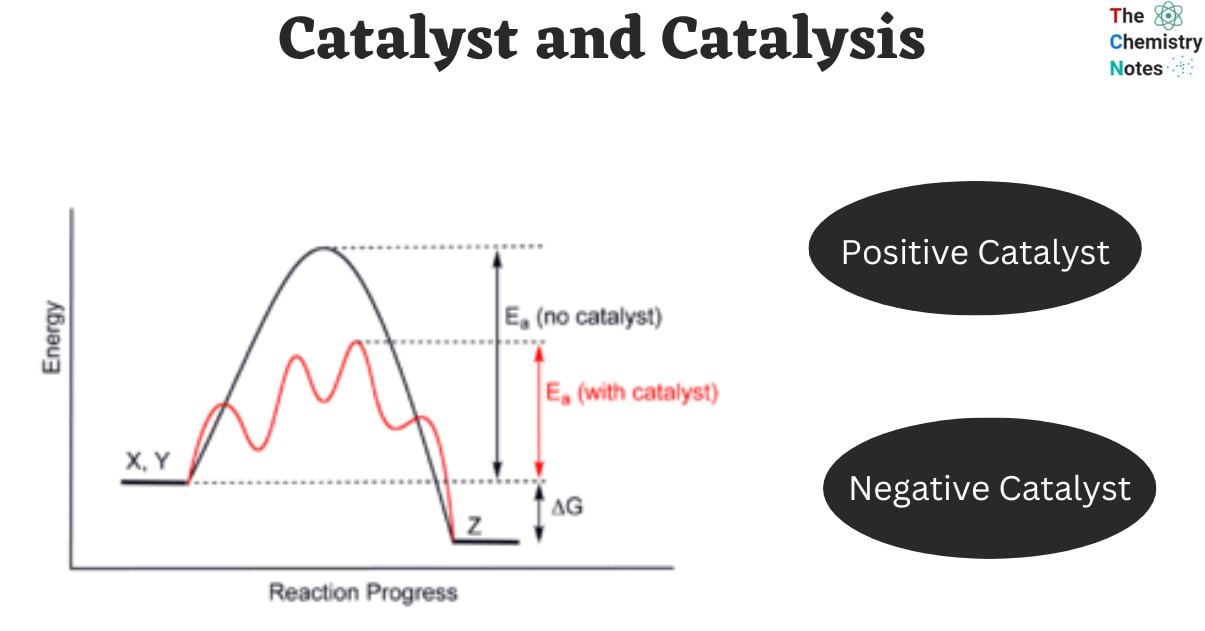
Catalyst Science Example . A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. Enzymes are naturally occurring catalysts responsible for many. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. There are four main types of catalysts: A catalyst is some material that speeds up chemical reactions. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. In heterogeneous catalysis, catalysts provide a surface to. Homogenous catalysts, heterogeneous catalysts, heterogenized homogenous catalysts, and biocatalysts. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Sometimes chemicals need a little.
from scienceinfo.com
Sometimes chemicals need a little. Homogenous catalysts, heterogeneous catalysts, heterogenized homogenous catalysts, and biocatalysts. A catalyst is some material that speeds up chemical reactions. In heterogeneous catalysis, catalysts provide a surface to. Enzymes are naturally occurring catalysts responsible for many. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. There are four main types of catalysts: A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds.
Catalyst and Catalysis Types, Examples, Differences
Catalyst Science Example Enzymes are naturally occurring catalysts responsible for many. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is some material that speeds up chemical reactions. There are four main types of catalysts: In heterogeneous catalysis, catalysts provide a surface to. Sometimes chemicals need a little. Enzymes are naturally occurring catalysts responsible for many. Homogenous catalysts, heterogeneous catalysts, heterogenized homogenous catalysts, and biocatalysts. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself.
From scienceinfo.com
Catalyst and Catalysis Types, Examples, Differences Catalyst Science Example There are four main types of catalysts: Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Sometimes chemicals need a little. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. With a helping hand from a catalyst, molecules that might take years to. Catalyst Science Example.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalyst Science Example Enzymes are naturally occurring catalysts responsible for many. In heterogeneous catalysis, catalysts provide a surface to. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. Homogenous catalysts, heterogeneous catalysts, heterogenized homogenous catalysts, and biocatalysts. Sometimes chemicals need a little. Catalyst, in chemistry, any substance that increases. Catalyst Science Example.
From www.slideshare.net
Biology 2.4 Catalyst Science Example Sometimes chemicals need a little. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Enzymes are naturally occurring catalysts responsible for many. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. In heterogeneous catalysis, catalysts provide a surface to. A catalyst is. Catalyst Science Example.
From www.youtube.com
How does a CATALYST work ? YouTube Catalyst Science Example Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Enzymes are naturally occurring catalysts responsible for many. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. There are four main types of catalysts: Sometimes chemicals need. Catalyst Science Example.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Catalyst Science Example Homogenous catalysts, heterogeneous catalysts, heterogenized homogenous catalysts, and biocatalysts. Enzymes are naturally occurring catalysts responsible for many. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. In heterogeneous catalysis, catalysts. Catalyst Science Example.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Catalyst Science Example Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. Homogenous catalysts, heterogeneous catalysts, heterogenized homogenous catalysts, and biocatalysts. In heterogeneous catalysis, catalysts provide a surface to. There. Catalyst Science Example.
From www.slideserve.com
PPT IGCSE Chemistry PowerPoint Presentation, free download ID3496562 Catalyst Science Example Homogenous catalysts, heterogeneous catalysts, heterogenized homogenous catalysts, and biocatalysts. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. Enzymes are naturally occurring catalysts responsible for many. Sometimes chemicals need a little. A catalyst is some material that speeds up chemical reactions. In heterogeneous catalysis, catalysts provide. Catalyst Science Example.
From www.nagwa.com
Lesson Catalysts Nagwa Catalyst Science Example Sometimes chemicals need a little. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. A catalyst is some material that speeds up chemical reactions. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a substance that speeds up a chemical. Catalyst Science Example.
From www.pinterest.com
Catalyst Easy Science Energy activities, Chemical reactions Catalyst Science Example A catalyst is some material that speeds up chemical reactions. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. In heterogeneous catalysis, catalysts provide a surface to. Enzymes are naturally. Catalyst Science Example.
From www.slideserve.com
PPT Catalysts PowerPoint Presentation, free download ID2380033 Catalyst Science Example A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. Enzymes are naturally occurring catalysts responsible for many. In heterogeneous catalysis, catalysts provide a surface to. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts allow a reaction to. Catalyst Science Example.
From www.tes.com
Catalysts lesson and experiment Teaching Resources Catalyst Science Example Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. There are four main types of catalysts: Homogenous catalysts, heterogeneous catalysts, heterogenized homogenous catalysts, and biocatalysts. Enzymes are naturally occurring catalysts responsible for many. In heterogeneous catalysis, catalysts provide a surface to. Sometimes chemicals need a little. With a helping hand from a catalyst,. Catalyst Science Example.
From www.mdpi.com
Catalysts Free FullText System Chemistry in Catalysis Facing the Catalyst Science Example Enzymes are naturally occurring catalysts responsible for many. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. There are four main types of catalysts: In heterogeneous catalysis, catalysts provide a surface to. A catalyst is some material that speeds up chemical reactions. Homogenous catalysts, heterogeneous catalysts, heterogenized homogenous catalysts, and. Catalyst Science Example.
From www.slideserve.com
PPT Starter 1)Definition of catalysts 2) Difference between Catalyst Science Example Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Sometimes chemicals need a little. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than. Catalyst Science Example.
From www.slideserve.com
PPT Catalysts PowerPoint Presentation, free download ID2684800 Catalyst Science Example In heterogeneous catalysis, catalysts provide a surface to. Sometimes chemicals need a little. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Homogenous catalysts, heterogeneous catalysts, heterogenized homogenous catalysts, and biocatalysts. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. There are four. Catalyst Science Example.
From www.slideteam.net
Catalyst Reaction With Bubble And Beakers Template Presentation Catalyst Science Example Homogenous catalysts, heterogeneous catalysts, heterogenized homogenous catalysts, and biocatalysts. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. Sometimes chemicals need a little. There are four main types of catalysts: A catalyst is some material that speeds up chemical reactions. In heterogeneous catalysis, catalysts provide a. Catalyst Science Example.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalyst Science Example Sometimes chemicals need a little. Enzymes are naturally occurring catalysts responsible for many. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. With a helping hand from a catalyst, molecules. Catalyst Science Example.
From www.ck12.org
Catalysts Example 1 ( Video ) Chemistry CK12 Foundation Catalyst Science Example Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. There are four main types of catalysts: Enzymes are naturally occurring catalysts responsible for many. In heterogeneous catalysis, catalysts provide a surface to. With a helping hand from a catalyst, molecules that might take years to interact can now do so. Catalyst Science Example.
From www.flickr.com
H2 Catalyst The recordbreaking catalyst stuffs electrons … Flickr Catalyst Science Example Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. In heterogeneous catalysis, catalysts provide a surface to. A catalyst is some material that speeds up chemical reactions. Sometimes chemicals need. Catalyst Science Example.
From www.youtube.com
Positive & Negative Catalyst Types of Catalyst Class 9th & 11th Catalyst Science Example Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. Homogenous catalysts, heterogeneous catalysts, heterogenized homogenous catalysts, and biocatalysts. There are four main types of catalysts: Sometimes chemicals. Catalyst Science Example.
From examples.yourdictionary.com
Examples of Catalysts YourDictionary Catalyst Science Example There are four main types of catalysts: A catalyst is some material that speeds up chemical reactions. Enzymes are naturally occurring catalysts responsible for many. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Homogenous catalysts, heterogeneous catalysts, heterogenized homogenous catalysts, and biocatalysts. In heterogeneous catalysis, catalysts provide a surface to. Sometimes chemicals. Catalyst Science Example.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalyst Science Example A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Homogenous catalysts, heterogeneous catalysts, heterogenized homogenous catalysts, and biocatalysts. With a helping hand from a catalyst, molecules that. Catalyst Science Example.
From www.youtube.com
Heterogeneous catalyst & catalysis 12th Std Chemistry Science Catalyst Science Example With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. Homogenous catalysts, heterogeneous catalysts, heterogenized homogenous catalysts, and biocatalysts. In heterogeneous catalysis, catalysts provide a surface to. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalyst, in chemistry, any. Catalyst Science Example.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst Catalyst Science Example In heterogeneous catalysis, catalysts provide a surface to. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. A catalyst is some material that speeds up chemical reactions. Homogenous catalysts, heterogeneous catalysts, heterogenized homogenous catalysts, and biocatalysts. Sometimes chemicals need a little. Enzymes are naturally occurring catalysts responsible for many.. Catalyst Science Example.
From www.science.org
Using nature’s blueprint to expand catalysis with Earthabundant metals Catalyst Science Example With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. There are four main types of catalysts: Sometimes chemicals need a little. In heterogeneous catalysis, catalysts provide a surface to. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. A. Catalyst Science Example.
From medicaltaste.weebly.com
Periodic table catalyst definition chemistry medicaltaste Catalyst Science Example Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. There are four main types of catalysts: Homogenous catalysts, heterogeneous catalysts, heterogenized homogenous catalysts, and biocatalysts. A catalyst is some material. Catalyst Science Example.
From www.pinterest.com
Homogeneous Catalyst Easy Science Ap chemistry, Chemical equation Catalyst Science Example Sometimes chemicals need a little. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Enzymes are naturally occurring catalysts responsible for many. There are four main types of catalysts: With. Catalyst Science Example.
From scitechdaily.com
Science Made Simple What Are Catalysts? Catalyst Science Example Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Homogenous catalysts, heterogeneous catalysts, heterogenized homogenous catalysts, and biocatalysts. Sometimes chemicals need a little. In heterogeneous catalysis, catalysts provide a surface to. There are four main types of catalysts: With a helping hand from a catalyst, molecules that might take years to interact can. Catalyst Science Example.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free Catalyst Science Example In heterogeneous catalysis, catalysts provide a surface to. Homogenous catalysts, heterogeneous catalysts, heterogenized homogenous catalysts, and biocatalysts. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. There. Catalyst Science Example.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 Catalyst Science Example Sometimes chemicals need a little. Enzymes are naturally occurring catalysts responsible for many. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is some material that speeds up chemical reactions. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one,. Catalyst Science Example.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalyst Science Example With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. Homogenous catalysts, heterogeneous catalysts, heterogenized homogenous catalysts, and biocatalysts. Sometimes chemicals need a little. There are four main types of catalysts: A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to. Catalyst Science Example.
From www.britannica.com
Catalyst Examples, Definition, & Facts Britannica Catalyst Science Example Homogenous catalysts, heterogeneous catalysts, heterogenized homogenous catalysts, and biocatalysts. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself. With a helping hand from a catalyst, molecules that. Catalyst Science Example.
From www.youtube.com
Demonstration of a Simple Catalyst Experiment YouTube Catalyst Science Example Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. In heterogeneous catalysis, catalysts provide a surface to. Homogenous catalysts, heterogeneous catalysts, heterogenized homogenous catalysts, and biocatalysts. There are four main types of catalysts: A catalyst is some material that speeds up chemical reactions. Enzymes are naturally occurring catalysts responsible for many. Catalysts allow. Catalyst Science Example.
From www.science.org
Tricks for noncovalent catalysis Science Catalyst Science Example There are four main types of catalysts: Homogenous catalysts, heterogeneous catalysts, heterogenized homogenous catalysts, and biocatalysts. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. Catalysts allow a reaction to proceed via a. Catalyst Science Example.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation ID1803655 Catalyst Science Example With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is some material that speeds up chemical reactions. Sometimes chemicals need a little. Homogenous catalysts, heterogeneous catalysts, heterogenized homogenous catalysts, and biocatalysts.. Catalyst Science Example.
From www.haikudeck.com
Project by Jees Hhss Catalyst Science Example Homogenous catalysts, heterogeneous catalysts, heterogenized homogenous catalysts, and biocatalysts. Enzymes are naturally occurring catalysts responsible for many. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. With a helping hand from a catalyst, molecules. Catalyst Science Example.