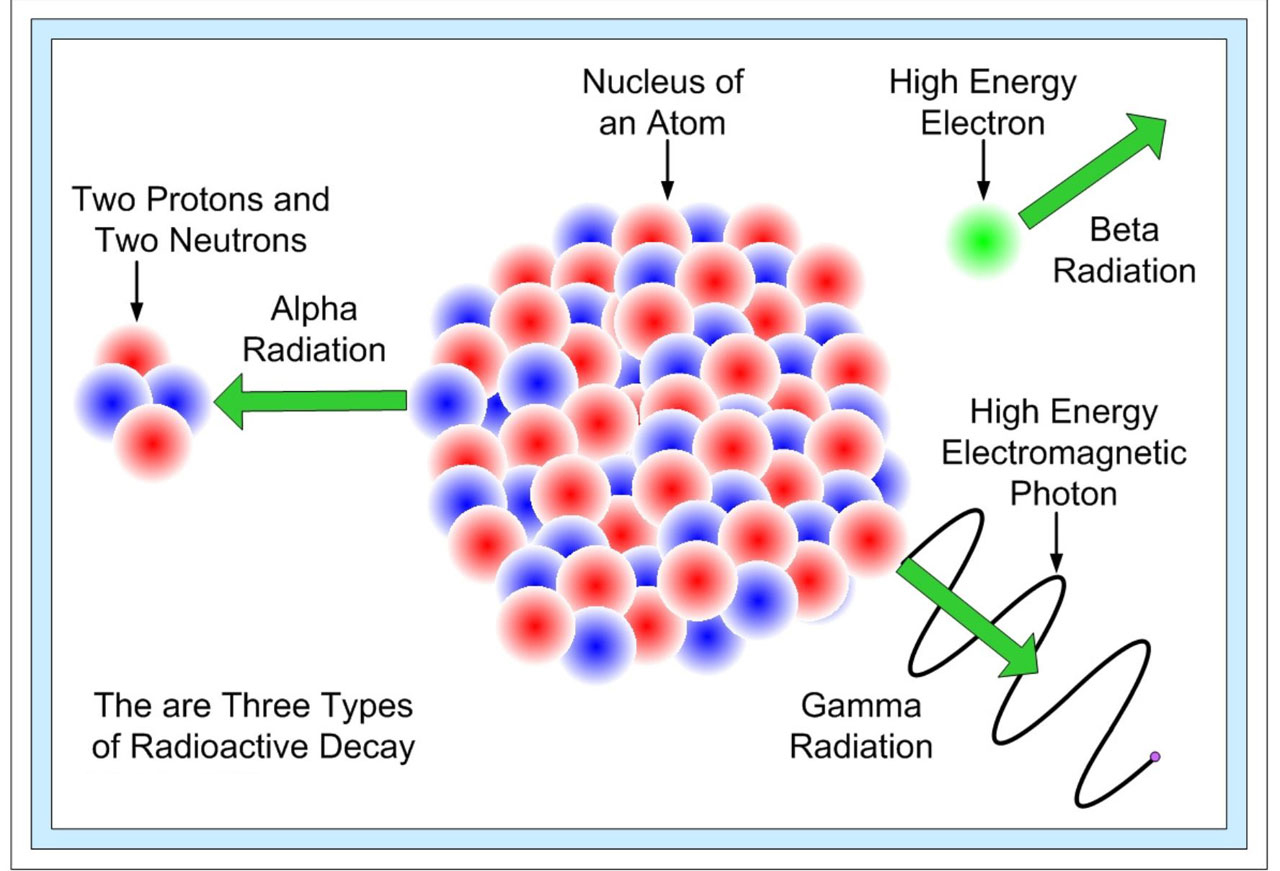
Radioactive And Non Radioactive Isotopes . Radioactive decay occurs when a radioactive atom gives off radiation in the form of energy or particles. Difference between stable and unstable. The main difference between the two is their stability. Radioactive isotopes are used in some materials inspection processes and the measurement of thicknesses, densities and. Radioisotopes are unstable and undergo radioactive decay, emitting radiation in the. Some isotopes are unstable and emit radiation in the form of particles and energy to form more stable elements. Radioactive isotope, any of several species of the same chemical element with different masses whose nuclei are unstable and dissipate excess. These are called radioactive isotopes or radioisotopes (figure 17.1.b 17.1. Some forms of radiation are dangerous. There are four types of radiation given off by radioactive atoms. Some elements have no stable isotopes, which means that any atom of that element is radioactive. Radioactive atoms give off radiation to become more stable. For some other elements, only certain isotopes are radioactive.
from www.edukamer.info
Difference between stable and unstable. The main difference between the two is their stability. Radioactive isotopes are used in some materials inspection processes and the measurement of thicknesses, densities and. For some other elements, only certain isotopes are radioactive. Radioactive atoms give off radiation to become more stable. There are four types of radiation given off by radioactive atoms. Radioisotopes are unstable and undergo radioactive decay, emitting radiation in the. Some forms of radiation are dangerous. These are called radioactive isotopes or radioisotopes (figure 17.1.b 17.1. Some elements have no stable isotopes, which means that any atom of that element is radioactive.
What is radioactivity? — Edukamer
Radioactive And Non Radioactive Isotopes Radioactive isotope, any of several species of the same chemical element with different masses whose nuclei are unstable and dissipate excess. Radioactive decay occurs when a radioactive atom gives off radiation in the form of energy or particles. Radioactive isotopes are used in some materials inspection processes and the measurement of thicknesses, densities and. The main difference between the two is their stability. Some forms of radiation are dangerous. Difference between stable and unstable. Radioisotopes are unstable and undergo radioactive decay, emitting radiation in the. For some other elements, only certain isotopes are radioactive. These are called radioactive isotopes or radioisotopes (figure 17.1.b 17.1. Radioactive isotope, any of several species of the same chemical element with different masses whose nuclei are unstable and dissipate excess. Some isotopes are unstable and emit radiation in the form of particles and energy to form more stable elements. Some elements have no stable isotopes, which means that any atom of that element is radioactive. There are four types of radiation given off by radioactive atoms. Radioactive atoms give off radiation to become more stable.
From www.slideserve.com
PPT Chemistry in Biology PowerPoint Presentation, free download ID Radioactive And Non Radioactive Isotopes Radioisotopes are unstable and undergo radioactive decay, emitting radiation in the. Radioactive atoms give off radiation to become more stable. For some other elements, only certain isotopes are radioactive. These are called radioactive isotopes or radioisotopes (figure 17.1.b 17.1. Some isotopes are unstable and emit radiation in the form of particles and energy to form more stable elements. Radioactive decay. Radioactive And Non Radioactive Isotopes.
From www.snexplores.org
Explainer Radiation and radioactive decay Radioactive And Non Radioactive Isotopes Some forms of radiation are dangerous. Some isotopes are unstable and emit radiation in the form of particles and energy to form more stable elements. There are four types of radiation given off by radioactive atoms. Radioactive atoms give off radiation to become more stable. Radioactive isotopes are used in some materials inspection processes and the measurement of thicknesses, densities. Radioactive And Non Radioactive Isotopes.
From ayda.net
Radioactive Elements in Periodic Table • Ayda Walsh Radioactive And Non Radioactive Isotopes Some forms of radiation are dangerous. These are called radioactive isotopes or radioisotopes (figure 17.1.b 17.1. For some other elements, only certain isotopes are radioactive. There are four types of radiation given off by radioactive atoms. Radioactive decay occurs when a radioactive atom gives off radiation in the form of energy or particles. Difference between stable and unstable. Radioisotopes are. Radioactive And Non Radioactive Isotopes.
From slideplayer.com
Unit Nuclear Chemistry ppt download Radioactive And Non Radioactive Isotopes Radioactive isotope, any of several species of the same chemical element with different masses whose nuclei are unstable and dissipate excess. Some isotopes are unstable and emit radiation in the form of particles and energy to form more stable elements. Radioactive isotopes are used in some materials inspection processes and the measurement of thicknesses, densities and. Some forms of radiation. Radioactive And Non Radioactive Isotopes.
From www.slideserve.com
PPT Chapter 4 Nuclear Chemistry PowerPoint Presentation, free Radioactive And Non Radioactive Isotopes Radioactive isotopes are used in some materials inspection processes and the measurement of thicknesses, densities and. There are four types of radiation given off by radioactive atoms. Some elements have no stable isotopes, which means that any atom of that element is radioactive. These are called radioactive isotopes or radioisotopes (figure 17.1.b 17.1. Some isotopes are unstable and emit radiation. Radioactive And Non Radioactive Isotopes.
From www.slideserve.com
PPT Atomic Nucleus and Radioactivity PowerPoint Presentation, free Radioactive And Non Radioactive Isotopes There are four types of radiation given off by radioactive atoms. Radioactive atoms give off radiation to become more stable. Some isotopes are unstable and emit radiation in the form of particles and energy to form more stable elements. Difference between stable and unstable. For some other elements, only certain isotopes are radioactive. These are called radioactive isotopes or radioisotopes. Radioactive And Non Radioactive Isotopes.
From www.slideserve.com
PPT Chapter 9 Nuclear Radiation PowerPoint Presentation, free Radioactive And Non Radioactive Isotopes Radioactive isotope, any of several species of the same chemical element with different masses whose nuclei are unstable and dissipate excess. For some other elements, only certain isotopes are radioactive. Difference between stable and unstable. These are called radioactive isotopes or radioisotopes (figure 17.1.b 17.1. Some elements have no stable isotopes, which means that any atom of that element is. Radioactive And Non Radioactive Isotopes.
From www.teachoo.com
Isotopes and Isobars Definition, Uses and Difference Teachoo Radioactive And Non Radioactive Isotopes Some elements have no stable isotopes, which means that any atom of that element is radioactive. Radioisotopes are unstable and undergo radioactive decay, emitting radiation in the. For some other elements, only certain isotopes are radioactive. Some forms of radiation are dangerous. Radioactive isotopes are used in some materials inspection processes and the measurement of thicknesses, densities and. Some isotopes. Radioactive And Non Radioactive Isotopes.
From timescavengers.blog
Isotopes Time Scavengers Radioactive And Non Radioactive Isotopes There are four types of radiation given off by radioactive atoms. Some isotopes are unstable and emit radiation in the form of particles and energy to form more stable elements. For some other elements, only certain isotopes are radioactive. Radioactive isotopes are used in some materials inspection processes and the measurement of thicknesses, densities and. Radioactive isotope, any of several. Radioactive And Non Radioactive Isotopes.
From www.dreamstime.com
Carbon Isotopes. Atom Structure Stock Vector Illustration of molecule Radioactive And Non Radioactive Isotopes Difference between stable and unstable. Radioactive isotopes are used in some materials inspection processes and the measurement of thicknesses, densities and. For some other elements, only certain isotopes are radioactive. Radioactive decay occurs when a radioactive atom gives off radiation in the form of energy or particles. The main difference between the two is their stability. Radioisotopes are unstable and. Radioactive And Non Radioactive Isotopes.
From www.pinterest.com
Difference Between Isotope and Radioisotope Definition, Properties Radioactive And Non Radioactive Isotopes The main difference between the two is their stability. For some other elements, only certain isotopes are radioactive. Radioisotopes are unstable and undergo radioactive decay, emitting radiation in the. Radioactive decay occurs when a radioactive atom gives off radiation in the form of energy or particles. Some elements have no stable isotopes, which means that any atom of that element. Radioactive And Non Radioactive Isotopes.
From www.slideserve.com
PPT Atomic Nucleus and Radioactivity PowerPoint Presentation, free Radioactive And Non Radioactive Isotopes Some elements have no stable isotopes, which means that any atom of that element is radioactive. These are called radioactive isotopes or radioisotopes (figure 17.1.b 17.1. Some forms of radiation are dangerous. The main difference between the two is their stability. Difference between stable and unstable. Radioactive atoms give off radiation to become more stable. Radioactive isotopes are used in. Radioactive And Non Radioactive Isotopes.
From courses.lumenlearning.com
Radioactive Decay Chemistry Radioactive And Non Radioactive Isotopes Radioactive isotopes are used in some materials inspection processes and the measurement of thicknesses, densities and. The main difference between the two is their stability. Some elements have no stable isotopes, which means that any atom of that element is radioactive. Radioactive decay occurs when a radioactive atom gives off radiation in the form of energy or particles. Some forms. Radioactive And Non Radioactive Isotopes.
From klakqcqlt.blob.core.windows.net
Non Radioactive Isotopes Of Cobalt at Penny Raposa blog Radioactive And Non Radioactive Isotopes Radioactive isotope, any of several species of the same chemical element with different masses whose nuclei are unstable and dissipate excess. There are four types of radiation given off by radioactive atoms. For some other elements, only certain isotopes are radioactive. Some elements have no stable isotopes, which means that any atom of that element is radioactive. Radioactive isotopes are. Radioactive And Non Radioactive Isotopes.
From www.expii.com
Isotopes — Definition & Overview Expii Radioactive And Non Radioactive Isotopes These are called radioactive isotopes or radioisotopes (figure 17.1.b 17.1. The main difference between the two is their stability. Radioactive atoms give off radiation to become more stable. Radioactive isotopes are used in some materials inspection processes and the measurement of thicknesses, densities and. Difference between stable and unstable. Some isotopes are unstable and emit radiation in the form of. Radioactive And Non Radioactive Isotopes.
From www.slideserve.com
PPT Minerals PowerPoint Presentation, free download ID2316086 Radioactive And Non Radioactive Isotopes Some forms of radiation are dangerous. The main difference between the two is their stability. Some elements have no stable isotopes, which means that any atom of that element is radioactive. Some isotopes are unstable and emit radiation in the form of particles and energy to form more stable elements. Radioactive isotope, any of several species of the same chemical. Radioactive And Non Radioactive Isotopes.
From www.slideserve.com
PPT Chapter 2 The Chemical Context of Life PowerPoint Presentation Radioactive And Non Radioactive Isotopes Some isotopes are unstable and emit radiation in the form of particles and energy to form more stable elements. Radioactive isotope, any of several species of the same chemical element with different masses whose nuclei are unstable and dissipate excess. There are four types of radiation given off by radioactive atoms. Difference between stable and unstable. The main difference between. Radioactive And Non Radioactive Isotopes.
From facts.net
16 Captivating Facts About Radioactive Isotope Radioactive And Non Radioactive Isotopes Radioisotopes are unstable and undergo radioactive decay, emitting radiation in the. Some forms of radiation are dangerous. Radioactive isotopes are used in some materials inspection processes and the measurement of thicknesses, densities and. The main difference between the two is their stability. Radioactive isotope, any of several species of the same chemical element with different masses whose nuclei are unstable. Radioactive And Non Radioactive Isotopes.
From www.yaclass.in
Radiochemistry and its applications — lesson. Science State Board, Class 9. Radioactive And Non Radioactive Isotopes Radioisotopes are unstable and undergo radioactive decay, emitting radiation in the. Difference between stable and unstable. Some elements have no stable isotopes, which means that any atom of that element is radioactive. Radioactive decay occurs when a radioactive atom gives off radiation in the form of energy or particles. The main difference between the two is their stability. These are. Radioactive And Non Radioactive Isotopes.
From joipffibu.blob.core.windows.net
What Are Two Ways Radioactive Isotopes Decay at Nancy Galindo blog Radioactive And Non Radioactive Isotopes There are four types of radiation given off by radioactive atoms. Difference between stable and unstable. For some other elements, only certain isotopes are radioactive. Radioactive isotopes are used in some materials inspection processes and the measurement of thicknesses, densities and. Radioactive atoms give off radiation to become more stable. Some forms of radiation are dangerous. Radioactive isotope, any of. Radioactive And Non Radioactive Isotopes.
From klakqcqlt.blob.core.windows.net
Non Radioactive Isotopes Of Cobalt at Penny Raposa blog Radioactive And Non Radioactive Isotopes Radioactive atoms give off radiation to become more stable. Radioactive decay occurs when a radioactive atom gives off radiation in the form of energy or particles. Difference between stable and unstable. These are called radioactive isotopes or radioisotopes (figure 17.1.b 17.1. Some isotopes are unstable and emit radiation in the form of particles and energy to form more stable elements.. Radioactive And Non Radioactive Isotopes.
From www.epa.gov
Radioactive Decay Radiation Protection US EPA Radioactive And Non Radioactive Isotopes Radioactive isotopes are used in some materials inspection processes and the measurement of thicknesses, densities and. Radioactive isotope, any of several species of the same chemical element with different masses whose nuclei are unstable and dissipate excess. There are four types of radiation given off by radioactive atoms. Radioisotopes are unstable and undergo radioactive decay, emitting radiation in the. Radioactive. Radioactive And Non Radioactive Isotopes.
From joidxtphp.blob.core.windows.net
What Are Two Uses Of Radioactive Isotopes at Steven Flanagan blog Radioactive And Non Radioactive Isotopes Some elements have no stable isotopes, which means that any atom of that element is radioactive. For some other elements, only certain isotopes are radioactive. Some isotopes are unstable and emit radiation in the form of particles and energy to form more stable elements. Radioactive decay occurs when a radioactive atom gives off radiation in the form of energy or. Radioactive And Non Radioactive Isotopes.
From slideplayer.com
TOPIC 1 MATTER AND THE THEORY OF STATE MATTER ppt download Radioactive And Non Radioactive Isotopes For some other elements, only certain isotopes are radioactive. Some elements have no stable isotopes, which means that any atom of that element is radioactive. Radioactive atoms give off radiation to become more stable. Some isotopes are unstable and emit radiation in the form of particles and energy to form more stable elements. Radioactive decay occurs when a radioactive atom. Radioactive And Non Radioactive Isotopes.
From sciencenotes.org
Radioactivity and the Types of Radioactive Decay Radioactive And Non Radioactive Isotopes Difference between stable and unstable. The main difference between the two is their stability. Radioactive isotope, any of several species of the same chemical element with different masses whose nuclei are unstable and dissipate excess. Radioactive decay occurs when a radioactive atom gives off radiation in the form of energy or particles. Radioactive isotopes are used in some materials inspection. Radioactive And Non Radioactive Isotopes.
From www.edukamer.info
What is radioactivity? — Edukamer Radioactive And Non Radioactive Isotopes Some elements have no stable isotopes, which means that any atom of that element is radioactive. For some other elements, only certain isotopes are radioactive. Radioactive atoms give off radiation to become more stable. Some forms of radiation are dangerous. Radioactive decay occurs when a radioactive atom gives off radiation in the form of energy or particles. Radioactive isotopes are. Radioactive And Non Radioactive Isotopes.
From www.slideserve.com
PPT Unit 3 Atomic Structure PowerPoint Presentation, free download Radioactive And Non Radioactive Isotopes Radioactive decay occurs when a radioactive atom gives off radiation in the form of energy or particles. Some elements have no stable isotopes, which means that any atom of that element is radioactive. Radioactive atoms give off radiation to become more stable. These are called radioactive isotopes or radioisotopes (figure 17.1.b 17.1. The main difference between the two is their. Radioactive And Non Radioactive Isotopes.
From www.geeksforgeeks.org
Radioactive Elements History, Examples, List, and Applications Radioactive And Non Radioactive Isotopes Radioactive atoms give off radiation to become more stable. Some forms of radiation are dangerous. Radioisotopes are unstable and undergo radioactive decay, emitting radiation in the. There are four types of radiation given off by radioactive atoms. Some elements have no stable isotopes, which means that any atom of that element is radioactive. The main difference between the two is. Radioactive And Non Radioactive Isotopes.
From www.thoughtco.com
A List of Radioactive Elements Radioactive And Non Radioactive Isotopes Radioactive decay occurs when a radioactive atom gives off radiation in the form of energy or particles. For some other elements, only certain isotopes are radioactive. There are four types of radiation given off by radioactive atoms. The main difference between the two is their stability. Some isotopes are unstable and emit radiation in the form of particles and energy. Radioactive And Non Radioactive Isotopes.
From www.scienceabc.com
Why Are Certain Elements Radioactive? » ScienceABC Radioactive And Non Radioactive Isotopes These are called radioactive isotopes or radioisotopes (figure 17.1.b 17.1. Some isotopes are unstable and emit radiation in the form of particles and energy to form more stable elements. Radioactive isotope, any of several species of the same chemical element with different masses whose nuclei are unstable and dissipate excess. Some elements have no stable isotopes, which means that any. Radioactive And Non Radioactive Isotopes.
From exocugzrn.blob.core.windows.net
Explain Nonradioactive at Heather Rhodes blog Radioactive And Non Radioactive Isotopes Difference between stable and unstable. Some isotopes are unstable and emit radiation in the form of particles and energy to form more stable elements. Radioactive isotopes are used in some materials inspection processes and the measurement of thicknesses, densities and. Radioactive atoms give off radiation to become more stable. Some elements have no stable isotopes, which means that any atom. Radioactive And Non Radioactive Isotopes.
From www.slideserve.com
PPT Chapter 39 The Atomic Nucleus and Radioactivity PowerPoint Radioactive And Non Radioactive Isotopes Some forms of radiation are dangerous. Difference between stable and unstable. Radioactive isotope, any of several species of the same chemical element with different masses whose nuclei are unstable and dissipate excess. The main difference between the two is their stability. Radioactive decay occurs when a radioactive atom gives off radiation in the form of energy or particles. Radioisotopes are. Radioactive And Non Radioactive Isotopes.
From klakqcqlt.blob.core.windows.net
Non Radioactive Isotopes Of Cobalt at Penny Raposa blog Radioactive And Non Radioactive Isotopes Some isotopes are unstable and emit radiation in the form of particles and energy to form more stable elements. There are four types of radiation given off by radioactive atoms. Difference between stable and unstable. Radioactive isotopes are used in some materials inspection processes and the measurement of thicknesses, densities and. For some other elements, only certain isotopes are radioactive.. Radioactive And Non Radioactive Isotopes.
From telegra.ph
What Is The Difference Between Radioactive Isotope And Radioactive Radioactive And Non Radioactive Isotopes Some isotopes are unstable and emit radiation in the form of particles and energy to form more stable elements. These are called radioactive isotopes or radioisotopes (figure 17.1.b 17.1. For some other elements, only certain isotopes are radioactive. The main difference between the two is their stability. Radioisotopes are unstable and undergo radioactive decay, emitting radiation in the. Radioactive atoms. Radioactive And Non Radioactive Isotopes.
From www.slideserve.com
PPT Matter and atomic structure PowerPoint Presentation, free Radioactive And Non Radioactive Isotopes For some other elements, only certain isotopes are radioactive. There are four types of radiation given off by radioactive atoms. Difference between stable and unstable. Some forms of radiation are dangerous. Radioisotopes are unstable and undergo radioactive decay, emitting radiation in the. These are called radioactive isotopes or radioisotopes (figure 17.1.b 17.1. Some isotopes are unstable and emit radiation in. Radioactive And Non Radioactive Isotopes.