Titration To Determine Ph . calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added. to calculate ph at any point in a titration, the amounts of all species must first be determined using the stoichiometry of the. The equivalence point of a titration. By the end of this section, you will be able to: By the end of this section, you will be able to: a graph of ph (column b) plotted as ordinate vs. in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. Volume (column a) as abscissa provides a titration curve as illustrated by graph 1.
from www.youtube.com
a graph of ph (column b) plotted as ordinate vs. By the end of this section, you will be able to: Volume (column a) as abscissa provides a titration curve as illustrated by graph 1. The equivalence point of a titration. to calculate ph at any point in a titration, the amounts of all species must first be determined using the stoichiometry of the. By the end of this section, you will be able to: in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added.
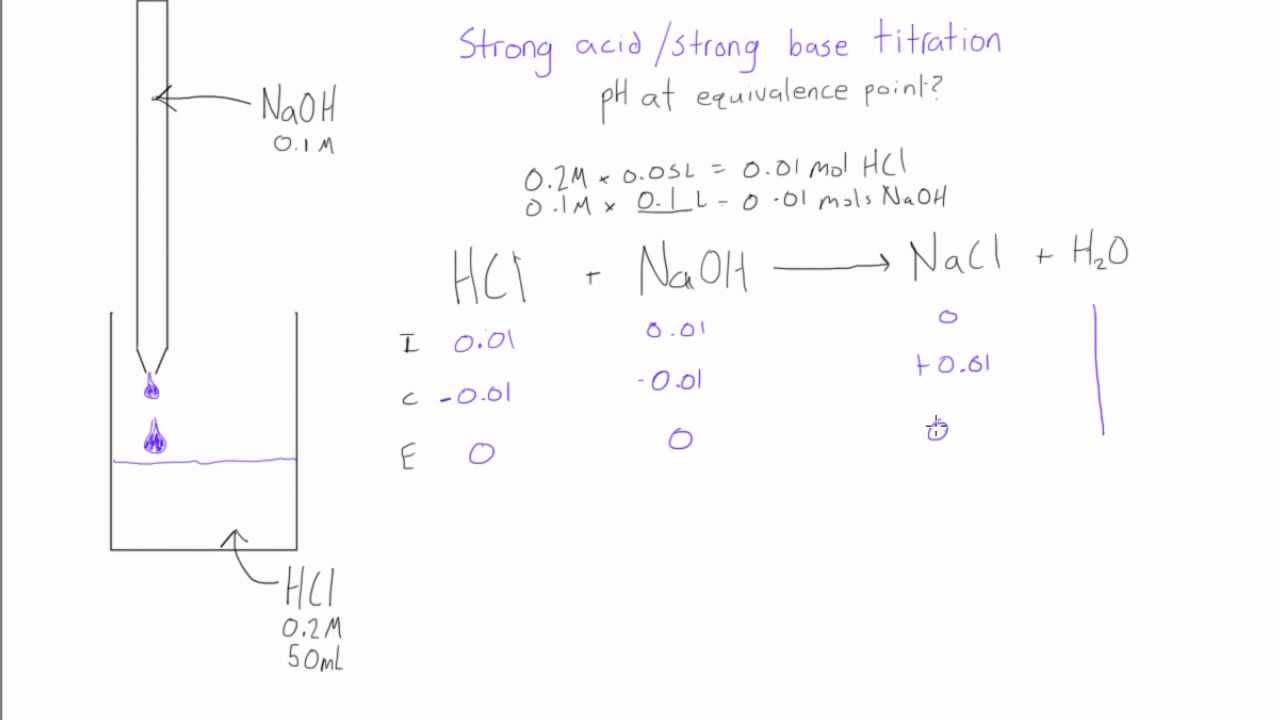
Strong acid / strong base titration pH at equivalence point YouTube
Titration To Determine Ph to calculate ph at any point in a titration, the amounts of all species must first be determined using the stoichiometry of the. in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. a graph of ph (column b) plotted as ordinate vs. The equivalence point of a titration. By the end of this section, you will be able to: calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added. to calculate ph at any point in a titration, the amounts of all species must first be determined using the stoichiometry of the. Volume (column a) as abscissa provides a titration curve as illustrated by graph 1. By the end of this section, you will be able to:
From mavink.com
Acid Base Titration Equation Titration To Determine Ph By the end of this section, you will be able to: calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added. By the end of this section, you will be able to: The equivalence point of a titration. a. Titration To Determine Ph.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration To Determine Ph By the end of this section, you will be able to: The equivalence point of a titration. to calculate ph at any point in a titration, the amounts of all species must first be determined using the stoichiometry of the. in this section we will learn how to calculate the ph of an analyte solution throughout the titration,. Titration To Determine Ph.
From www.youtube.com
Weak acid / strong base titration pH after equivalence point YouTube Titration To Determine Ph Volume (column a) as abscissa provides a titration curve as illustrated by graph 1. in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. to calculate ph at any point in a titration, the amounts of all species must first be determined using the stoichiometry of the.. Titration To Determine Ph.
From exoqbgfse.blob.core.windows.net
Sample Lab Report On Acid Base Titration at Michelle Hamilton blog Titration To Determine Ph in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. The equivalence point of a titration. a graph of ph (column b) plotted as ordinate vs. By the end of this section, you will be able to: Volume (column a) as abscissa provides a titration curve as. Titration To Determine Ph.
From www.coursehero.com
[Solved] Calculate the pH at the equivalence point for the titration... Course Hero Titration To Determine Ph to calculate ph at any point in a titration, the amounts of all species must first be determined using the stoichiometry of the. in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. By the end of this section, you will be able to: a graph. Titration To Determine Ph.
From chemwiki.ucdavis.edu
Titration of a Weak Base with a Strong Acid Chemwiki Titration To Determine Ph in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. By the end of this section, you will be able to: a graph of ph (column b) plotted as ordinate vs. The equivalence point of a titration. to calculate ph at any point in a titration,. Titration To Determine Ph.
From www.youtube.com
Acid Base Titrations, pH Curves and Endpoints YouTube Titration To Determine Ph a graph of ph (column b) plotted as ordinate vs. The equivalence point of a titration. to calculate ph at any point in a titration, the amounts of all species must first be determined using the stoichiometry of the. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq). Titration To Determine Ph.
From www.youtube.com
Weak acid / strong base titration pH at equivalence point YouTube Titration To Determine Ph in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. The equivalence point of a titration. a graph of ph (column b) plotted as ordinate vs. Volume (column a) as abscissa provides a titration curve as illustrated by graph 1. to calculate ph at any point. Titration To Determine Ph.
From chemistryguru.com.sg
Titration Curve for Weak Acid Strong Base Titration To Determine Ph By the end of this section, you will be able to: a graph of ph (column b) plotted as ordinate vs. Volume (column a) as abscissa provides a titration curve as illustrated by graph 1. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant). Titration To Determine Ph.
From knowledge.carolina.com
Titrations Techniques and Calculations Carolina Knowledge Center Titration To Determine Ph calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added. a graph of ph (column b) plotted as ordinate vs. to calculate ph at any point in a titration, the amounts of all species must first be determined. Titration To Determine Ph.
From mungfali.com
Ph Titration Curve Titration To Determine Ph Volume (column a) as abscissa provides a titration curve as illustrated by graph 1. in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. By the end of this section, you will be able to: a graph of ph (column b) plotted as ordinate vs. calculate. Titration To Determine Ph.
From saylordotorg.github.io
AcidBase Titrations Titration To Determine Ph Volume (column a) as abscissa provides a titration curve as illustrated by graph 1. The equivalence point of a titration. a graph of ph (column b) plotted as ordinate vs. in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. By the end of this section, you. Titration To Determine Ph.
From www.youtube.com
Acid Base Titration Curves pH Calculations YouTube Titration To Determine Ph a graph of ph (column b) plotted as ordinate vs. By the end of this section, you will be able to: in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. Volume (column a) as abscissa provides a titration curve as illustrated by graph 1. calculate. Titration To Determine Ph.
From www.youtube.com
Strong acid / strong base titration pH at equivalence point YouTube Titration To Determine Ph to calculate ph at any point in a titration, the amounts of all species must first be determined using the stoichiometry of the. By the end of this section, you will be able to: a graph of ph (column b) plotted as ordinate vs. The equivalence point of a titration. calculate the ph for the strong acid/strong. Titration To Determine Ph.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii Titration To Determine Ph to calculate ph at any point in a titration, the amounts of all species must first be determined using the stoichiometry of the. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added. a graph of ph (column. Titration To Determine Ph.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration To Determine Ph By the end of this section, you will be able to: calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added. to calculate ph at any point in a titration, the amounts of all species must first be determined. Titration To Determine Ph.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept Chemistry JoVe Titration To Determine Ph a graph of ph (column b) plotted as ordinate vs. Volume (column a) as abscissa provides a titration curve as illustrated by graph 1. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added. By the end of this. Titration To Determine Ph.
From www.youtube.com
Acid Base Equilibria Weak Acid Strong Base Titration. YouTube Titration To Determine Ph By the end of this section, you will be able to: By the end of this section, you will be able to: in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. The equivalence point of a titration. calculate the ph for the strong acid/strong base titration. Titration To Determine Ph.
From www.youtube.com
titration curve pH calculations YouTube Titration To Determine Ph Volume (column a) as abscissa provides a titration curve as illustrated by graph 1. to calculate ph at any point in a titration, the amounts of all species must first be determined using the stoichiometry of the. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m. Titration To Determine Ph.
From www.numerade.com
SOLVED Determine the pH during the titration of 66.3 mL of 0.483 M acetic acid (Ka = 1.8×105 Titration To Determine Ph calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added. By the end of this section, you will be able to: Volume (column a) as abscissa provides a titration curve as illustrated by graph 1. a graph of ph. Titration To Determine Ph.
From www.youtube.com
Using pH Meters for Simple Titrations YouTube Titration To Determine Ph in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. Volume (column a) as abscissa provides a titration curve as illustrated by graph 1. The equivalence point of a titration. a graph of ph (column b) plotted as ordinate vs. By the end of this section, you. Titration To Determine Ph.
From www.youtube.com
Calculations for Titration of Weak Base with Strong Acid, initial pH YouTube Titration To Determine Ph Volume (column a) as abscissa provides a titration curve as illustrated by graph 1. a graph of ph (column b) plotted as ordinate vs. to calculate ph at any point in a titration, the amounts of all species must first be determined using the stoichiometry of the. calculate the ph for the strong acid/strong base titration between. Titration To Determine Ph.
From www.easybiologyclass.com
What is Titration Curve? What is pKa? EasyBiologyClass Titration To Determine Ph The equivalence point of a titration. Volume (column a) as abscissa provides a titration curve as illustrated by graph 1. By the end of this section, you will be able to: calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of. Titration To Determine Ph.
From www.youtube.com
Calculating pH During Titration Reactions YouTube Titration To Determine Ph a graph of ph (column b) plotted as ordinate vs. By the end of this section, you will be able to: to calculate ph at any point in a titration, the amounts of all species must first be determined using the stoichiometry of the. By the end of this section, you will be able to: The equivalence point. Titration To Determine Ph.
From mmerevise.co.uk
pH Curves Questions and Revision MME Titration To Determine Ph to calculate ph at any point in a titration, the amounts of all species must first be determined using the stoichiometry of the. By the end of this section, you will be able to: Volume (column a) as abscissa provides a titration curve as illustrated by graph 1. The equivalence point of a titration. By the end of this. Titration To Determine Ph.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Titration To Determine Ph in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. By the end of this section, you will be able to: The equivalence point of a titration. a graph of ph (column b) plotted as ordinate vs. to calculate ph at any point in a titration,. Titration To Determine Ph.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Titration To Determine Ph By the end of this section, you will be able to: Volume (column a) as abscissa provides a titration curve as illustrated by graph 1. By the end of this section, you will be able to: The equivalence point of a titration. a graph of ph (column b) plotted as ordinate vs. calculate the ph for the strong. Titration To Determine Ph.
From mavink.com
Acid Base Titration Experiment Titration To Determine Ph By the end of this section, you will be able to: By the end of this section, you will be able to: The equivalence point of a titration. in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. calculate the ph for the strong acid/strong base titration. Titration To Determine Ph.
From www.youtube.com
Determining the pH During a Strong AcidWeak Base Titration at Equivalence YouTube Titration To Determine Ph By the end of this section, you will be able to: a graph of ph (column b) plotted as ordinate vs. in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. to calculate ph at any point in a titration, the amounts of all species must. Titration To Determine Ph.
From www.youtube.com
pH titration curve calculations for weak acid strong base YouTube Titration To Determine Ph By the end of this section, you will be able to: to calculate ph at any point in a titration, the amounts of all species must first be determined using the stoichiometry of the. a graph of ph (column b) plotted as ordinate vs. By the end of this section, you will be able to: in this. Titration To Determine Ph.
From www.reddit.com
in pH titration, how do you determine the (multiple) pKa points from the graph (drawn from Titration To Determine Ph Volume (column a) as abscissa provides a titration curve as illustrated by graph 1. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added. By the end of this section, you will be able to: By the end of this. Titration To Determine Ph.
From general.chemistrysteps.com
Titration of a Weak Acid by a Strong Base Chemistry Steps Titration To Determine Ph By the end of this section, you will be able to: a graph of ph (column b) plotted as ordinate vs. The equivalence point of a titration. Volume (column a) as abscissa provides a titration curve as illustrated by graph 1. to calculate ph at any point in a titration, the amounts of all species must first be. Titration To Determine Ph.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations, Examples, Solution Stoichiometry Titration To Determine Ph to calculate ph at any point in a titration, the amounts of all species must first be determined using the stoichiometry of the. The equivalence point of a titration. Volume (column a) as abscissa provides a titration curve as illustrated by graph 1. in this section we will learn how to calculate the ph of an analyte solution. Titration To Determine Ph.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation ID6592809 Titration To Determine Ph Volume (column a) as abscissa provides a titration curve as illustrated by graph 1. By the end of this section, you will be able to: By the end of this section, you will be able to: The equivalence point of a titration. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3. Titration To Determine Ph.
From www.slideserve.com
PPT Titration and pH Curves. PowerPoint Presentation, free download ID2145668 Titration To Determine Ph a graph of ph (column b) plotted as ordinate vs. to calculate ph at any point in a titration, the amounts of all species must first be determined using the stoichiometry of the. Volume (column a) as abscissa provides a titration curve as illustrated by graph 1. By the end of this section, you will be able to:. Titration To Determine Ph.