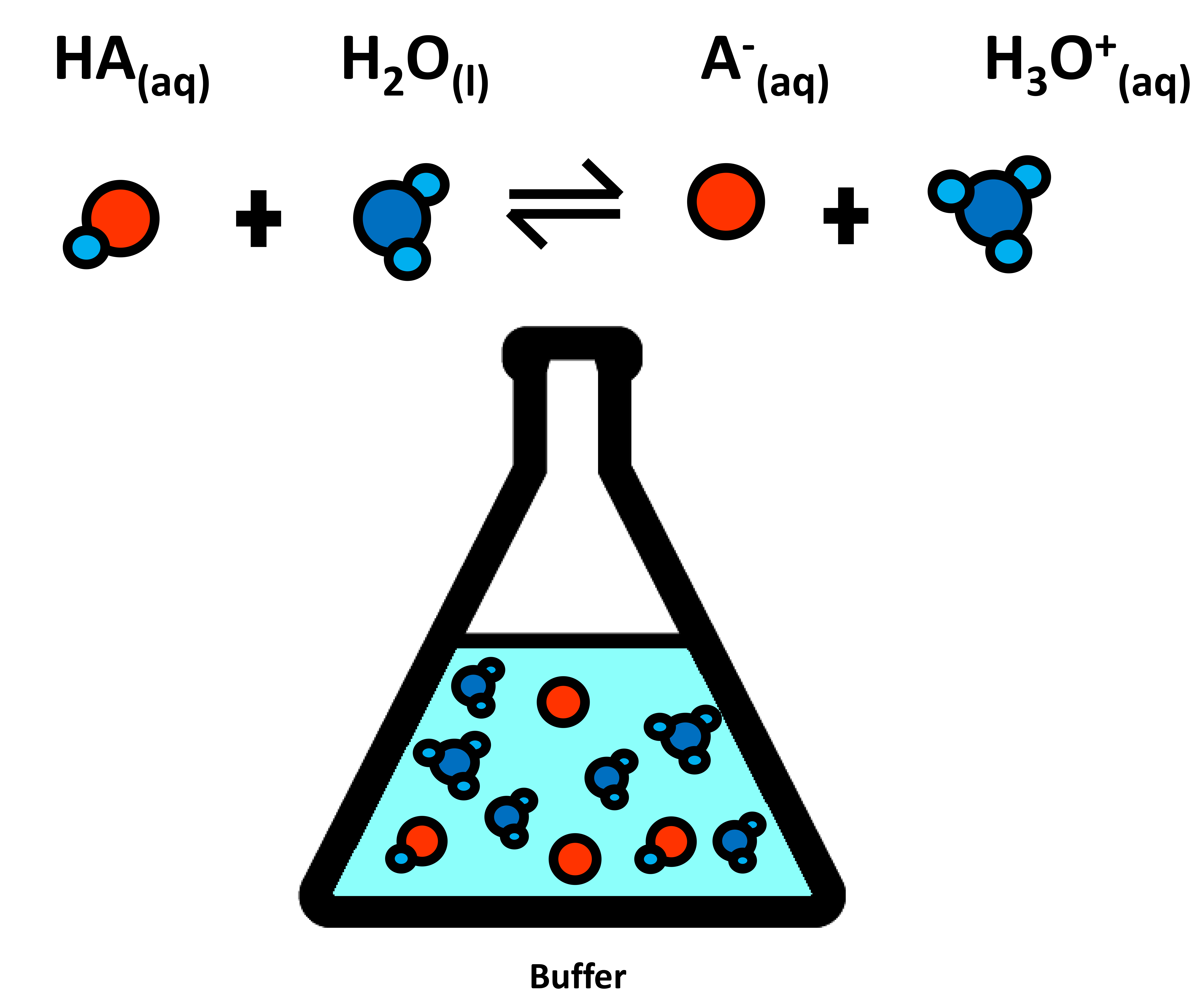
Ph Buffer Definition Biology . Buffers are important in biological. Buffers are important in biological. buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. if you're seeing this message, it means we're having trouble loading external resources on our website. buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. physiologic ph is a way of quantifying the balance between acids and bases in the body. Biological systems have peak activity in a very ph narrow range (at a ph of about 7 most of the time). If you're behind a web filter, please make sure that the domains. In fact, the ph depends on the concentration of hydrogen ions and can be. buffers in biology and biological buffers. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and sodium bicarbonate (na +.
from goldbio.com
If you're behind a web filter, please make sure that the domains. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and sodium bicarbonate (na +. buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. buffers in biology and biological buffers. In fact, the ph depends on the concentration of hydrogen ions and can be. Buffers are important in biological. physiologic ph is a way of quantifying the balance between acids and bases in the body. Buffers are important in biological. Biological systems have peak activity in a very ph narrow range (at a ph of about 7 most of the time).
What is a Biological Buffer and How to Choose the Best Buffer for Your
Ph Buffer Definition Biology Biological systems have peak activity in a very ph narrow range (at a ph of about 7 most of the time). if you're seeing this message, it means we're having trouble loading external resources on our website. In fact, the ph depends on the concentration of hydrogen ions and can be. buffers in biology and biological buffers. Biological systems have peak activity in a very ph narrow range (at a ph of about 7 most of the time). Buffers are important in biological. Buffers are important in biological. If you're behind a web filter, please make sure that the domains. buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and sodium bicarbonate (na +. physiologic ph is a way of quantifying the balance between acids and bases in the body.
From www.slideserve.com
PPT pH and Buffers PowerPoint Presentation, free download ID2948821 Ph Buffer Definition Biology vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and sodium bicarbonate (na +. if you're seeing this message, it means we're having trouble loading external resources on our website. Buffers are important in biological. buffers in biology and biological buffers. Buffers are important in. Ph Buffer Definition Biology.
From www.peoi.org
Chapter 12 Section G Buffers Ph Buffer Definition Biology buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. physiologic ph is a way of quantifying the balance between acids and bases in the body. Buffers are important in biological. If you're behind a web filter, please make sure that the domains. Buffers are important in biological. if. Ph Buffer Definition Biology.
From www.slideserve.com
PPT UNIT V pH, BUFFERS AND ISOTONIC SOLUTION PowerPoint Presentation Ph Buffer Definition Biology buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. if you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please make sure that the domains. buffers are solutions that moderate ph changes when an acid or. Ph Buffer Definition Biology.
From cebyatow.blob.core.windows.net
Buffer In Biology Definition at Rachel Ferrer blog Ph Buffer Definition Biology buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. Biological systems have peak activity in a very ph narrow range (at a ph of about 7 most of the time). buffers in biology and biological buffers. Buffers are important in biological. buffers are solutions that moderate ph changes. Ph Buffer Definition Biology.
From exohjgjqq.blob.core.windows.net
Buffer Biology Sentence at Valencia Douglas blog Ph Buffer Definition Biology physiologic ph is a way of quantifying the balance between acids and bases in the body. if you're seeing this message, it means we're having trouble loading external resources on our website. In fact, the ph depends on the concentration of hydrogen ions and can be. buffers in biology and biological buffers. buffers are solutions that. Ph Buffer Definition Biology.
From www.hopaxfc.com
How can Biological Buffer maintain the pH value? Blog Hopax Fine Ph Buffer Definition Biology if you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please make sure that the domains. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and sodium bicarbonate (na +. buffers are solutions. Ph Buffer Definition Biology.
From www.slideserve.com
PPT Biological Buffers PowerPoint Presentation, free download ID Ph Buffer Definition Biology if you're seeing this message, it means we're having trouble loading external resources on our website. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and sodium bicarbonate (na +. Buffers are important in biological. If you're behind a web filter, please make sure that the. Ph Buffer Definition Biology.
From www.youtube.com
Buffers and pH Biology YouTube Ph Buffer Definition Biology buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. if you're seeing this message, it means we're having trouble loading external resources on our website. Buffers are important in biological. If you're behind a web filter, please make sure that the domains. vertebrate organisms maintain the ph of. Ph Buffer Definition Biology.
From www.youtube.com
MB.1.2. Ph scale (HSC biology) YouTube Ph Buffer Definition Biology Buffers are important in biological. In fact, the ph depends on the concentration of hydrogen ions and can be. buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3). Ph Buffer Definition Biology.
From cezmyjhn.blob.core.windows.net
What Is The Purpose Of A Ph Buffer at Margaret Adams blog Ph Buffer Definition Biology Biological systems have peak activity in a very ph narrow range (at a ph of about 7 most of the time). buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. If you're behind a web filter, please make sure that the domains. In fact, the ph depends on the concentration. Ph Buffer Definition Biology.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Ph Buffer Definition Biology vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and sodium bicarbonate (na +. In fact, the ph depends on the concentration of hydrogen ions and can be. Buffers are important in biological. Biological systems have peak activity in a very ph narrow range (at a ph. Ph Buffer Definition Biology.
From www.worksheetsplanet.com
What is PH Definition Ph Buffer Definition Biology physiologic ph is a way of quantifying the balance between acids and bases in the body. if you're seeing this message, it means we're having trouble loading external resources on our website. In fact, the ph depends on the concentration of hydrogen ions and can be. Buffers are important in biological. buffers are solutions that moderate ph. Ph Buffer Definition Biology.
From derangedphysiology.com
Buffers and buffering power Deranged Physiology Ph Buffer Definition Biology buffers in biology and biological buffers. Buffers are important in biological. buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. In fact, the ph depends on the concentration of hydrogen. Ph Buffer Definition Biology.
From content.byui.edu
Acids, Bases, pH and Buffers Ph Buffer Definition Biology Buffers are important in biological. if you're seeing this message, it means we're having trouble loading external resources on our website. Buffers are important in biological. In fact, the ph depends on the concentration of hydrogen ions and can be. Biological systems have peak activity in a very ph narrow range (at a ph of about 7 most of. Ph Buffer Definition Biology.
From www.slideserve.com
PPT Cell Biology Cell Compounds and Biological Molecules PowerPoint Ph Buffer Definition Biology In fact, the ph depends on the concentration of hydrogen ions and can be. Buffers are important in biological. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and sodium bicarbonate (na +. Biological systems have peak activity in a very ph narrow range (at a ph. Ph Buffer Definition Biology.
From www.vrogue.co
How Do Buffers Work An Easy Explaination For Biologis vrogue.co Ph Buffer Definition Biology buffers in biology and biological buffers. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and sodium bicarbonate (na +. buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. Biological systems have peak activity in a. Ph Buffer Definition Biology.
From www.slideserve.com
PPT THEME Acidbase equilibrium in biological systems. Buffer Ph Buffer Definition Biology In fact, the ph depends on the concentration of hydrogen ions and can be. if you're seeing this message, it means we're having trouble loading external resources on our website. buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. physiologic ph is a way of quantifying the balance. Ph Buffer Definition Biology.
From www.slideserve.com
PPT pH and Buffers PowerPoint Presentation ID2948821 Ph Buffer Definition Biology if you're seeing this message, it means we're having trouble loading external resources on our website. Biological systems have peak activity in a very ph narrow range (at a ph of about 7 most of the time). buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. buffers in. Ph Buffer Definition Biology.
From exojbdfvv.blob.core.windows.net
What Is Biological Buffer System at Maureen Masters blog Ph Buffer Definition Biology buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. physiologic ph is a way of quantifying the balance between acids and bases in the body. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and sodium. Ph Buffer Definition Biology.
From cebyatow.blob.core.windows.net
Buffer In Biology Definition at Rachel Ferrer blog Ph Buffer Definition Biology If you're behind a web filter, please make sure that the domains. buffers in biology and biological buffers. buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. Buffers are important in biological. In fact, the ph depends on the concentration of hydrogen ions and can be. buffers are. Ph Buffer Definition Biology.
From goldbio.com
What is a Biological Buffer and How to Choose the Best Buffer for Your Ph Buffer Definition Biology buffers in biology and biological buffers. buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. In fact, the ph depends on the concentration of hydrogen ions and can be. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2. Ph Buffer Definition Biology.
From www.slideshare.net
Ph and buffer Ph Buffer Definition Biology buffers in biology and biological buffers. buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. Biological systems have peak activity in a very ph narrow range (at a ph of about 7 most of the time). if you're seeing this message, it means we're having trouble loading external. Ph Buffer Definition Biology.
From courses.lumenlearning.com
pH, Buffers, Acids, and Bases Introduction to Chemistry Ph Buffer Definition Biology buffers in biology and biological buffers. buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. If you're behind a web filter, please make sure that the domains. In fact, the ph depends on the concentration of hydrogen ions and can be. Biological systems have peak activity in a very. Ph Buffer Definition Biology.
From www.geeksforgeeks.org
Buffer Solution Definition, Types, Formula, Examples, and FAQs Ph Buffer Definition Biology buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. Buffers are important in biological. buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic. Ph Buffer Definition Biology.
From goldbio.com
What is a Biological Buffer and How to Choose the Best Buffer for Your Ph Buffer Definition Biology buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. If you're behind a web filter, please make sure that the domains. buffers in biology and biological buffers. buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. vertebrate organisms. Ph Buffer Definition Biology.
From www.slideserve.com
PPT Biological Buffers PowerPoint Presentation, free download ID Ph Buffer Definition Biology If you're behind a web filter, please make sure that the domains. Buffers are important in biological. In fact, the ph depends on the concentration of hydrogen ions and can be. buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. buffers in biology and biological buffers. buffers are. Ph Buffer Definition Biology.
From www.haikudeck.com
pH presentation (biology) by Colleen Doherty Ph Buffer Definition Biology vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and sodium bicarbonate (na +. buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. In fact, the ph depends on the concentration of hydrogen ions and can be.. Ph Buffer Definition Biology.
From www.youtube.com
Biochemistry Water, PH and Buffers Part 1 tutorial YouTube Ph Buffer Definition Biology buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and sodium bicarbonate (na +. physiologic ph is a way of quantifying the balance between acids and bases. Ph Buffer Definition Biology.
From www.youtube.com
Introduction to Buffer System Regulation of pH Acid Base Balance Ph Buffer Definition Biology In fact, the ph depends on the concentration of hydrogen ions and can be. physiologic ph is a way of quantifying the balance between acids and bases in the body. buffers in biology and biological buffers. buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. If you're behind. Ph Buffer Definition Biology.
From www.leinco.com
Biological Buffers And pH Range Leinco Technologies Ph Buffer Definition Biology physiologic ph is a way of quantifying the balance between acids and bases in the body. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and sodium bicarbonate (na +. buffers in biology and biological buffers. buffers are solutions that moderate ph changes when. Ph Buffer Definition Biology.
From lifeboat.com
Acids, Bases, PH, Buffers Ph Buffer Definition Biology Buffers are important in biological. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and sodium bicarbonate (na +. In fact, the ph depends on the concentration of hydrogen ions and can be. if you're seeing this message, it means we're having trouble loading external resources. Ph Buffer Definition Biology.
From cewwcrjd.blob.core.windows.net
Biological Buffers Ph Range at Milton Clark blog Ph Buffer Definition Biology buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. Buffers are important in biological. In fact, the ph depends on the concentration of hydrogen ions and can be. buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. physiologic ph. Ph Buffer Definition Biology.
From www.slideserve.com
PPT Acids, pH, and Buffers Some Basic Chemistry for Biological Ph Buffer Definition Biology buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. If you're behind a web filter, please make sure that the domains. Buffers are important in biological. In fact, the ph depends on the concentration of hydrogen ions and can be. if you're seeing this message, it means we're having. Ph Buffer Definition Biology.
From ceaaclpe.blob.core.windows.net
Buffer In Biology Example at Richard Durbin blog Ph Buffer Definition Biology buffers in biology and biological buffers. Biological systems have peak activity in a very ph narrow range (at a ph of about 7 most of the time). Buffers are important in biological. If you're behind a web filter, please make sure that the domains. physiologic ph is a way of quantifying the balance between acids and bases in. Ph Buffer Definition Biology.
From cebyatow.blob.core.windows.net
Buffer In Biology Definition at Rachel Ferrer blog Ph Buffer Definition Biology If you're behind a web filter, please make sure that the domains. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and sodium bicarbonate (na +. Buffers are important in biological. buffers in biology and biological buffers. if you're seeing this message, it means we're. Ph Buffer Definition Biology.